Home /
Expert Answers /
Chemistry /
for-the-following-equilibrium-system-which-of-the-following-changes-will-form-more-ca-oh-2-che-pa339
(Solved): For the following equilibrium system, which of the following changes will form more Ca(OH)_(2) ? Che ...
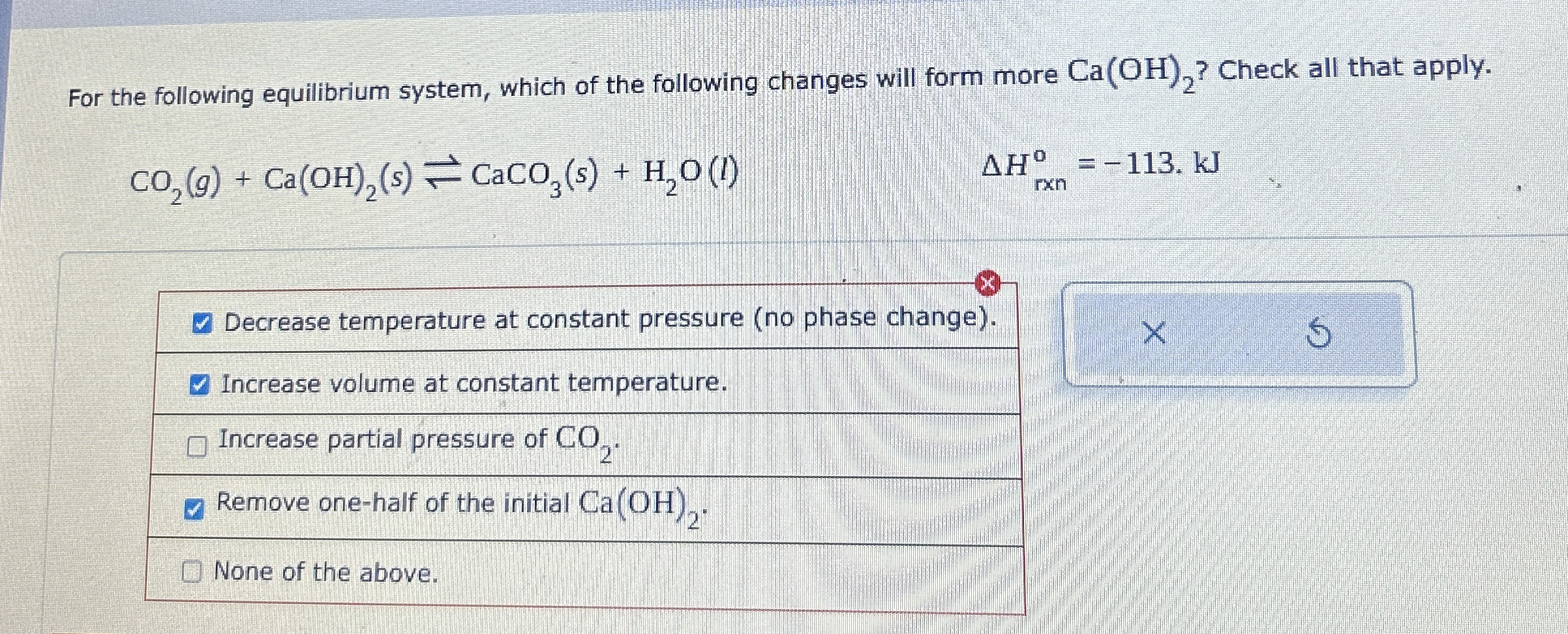
For the following equilibrium system, which of the following changes will form more
Ca(OH)_(2)? Check all that apply.
CO_(2)(g)+Ca(OH)_(2)(s)⇌CaCO_(3)(s)+H_(2)O(l),\Delta H_(rxn)^(o)=-113*kJDecrease temperature at constant pressure (no phase change). Increase volume at constant temperature. Increase partial pressure of
CO_(2). Remove one-half of the initial
Ca(OH)_(2). None of the above. For the following equilibrium system, which of the following changes will form more
Ca(OH)_(2)? Check all that apply.
CO_(2)(g)+Ca(OH)_(2)(s)⇌CaCO_(3)(s)+H_(2)O(l)
\Delta H_(rxn)\deg =-113.kJDecrease temperature at constant pressure (no phase change). Increase volume at constant temperature. Increase partial pressure of
CO_(2). Remove one-half of the initial
Ca(OH)_(2). None of the above.