Home /
Expert Answers /
Chemistry /
for-the-balanced-chemical-equation-below-mathrm-c-5-mathrm-h-12-8-mathrm-o-2-long-pa611
(Solved): For the balanced chemical equation below, \[ \mathrm{C}_{5} \mathrm{H}_{12}+8 \mathrm{O}_{2} \long ...
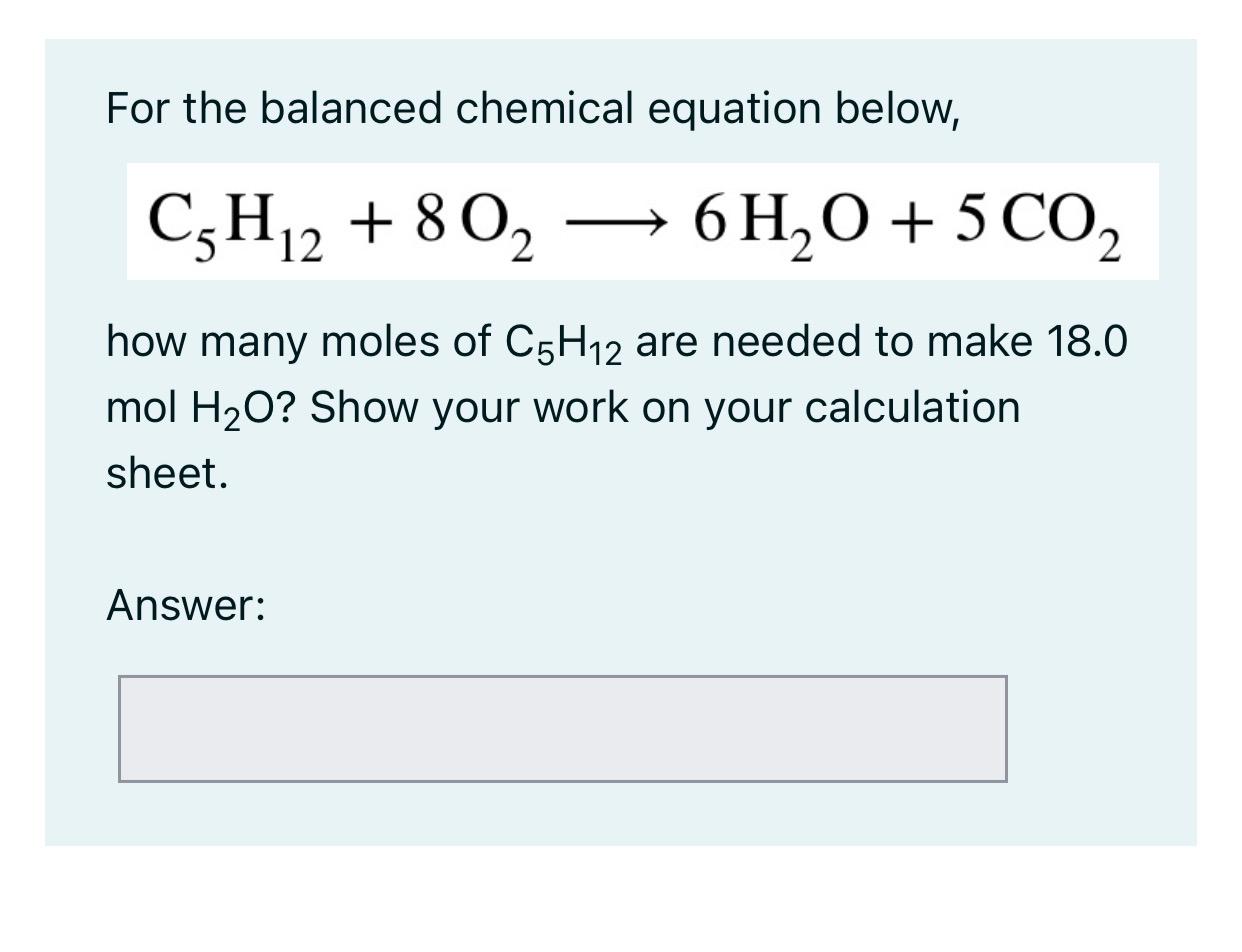
For the balanced chemical equation below, \[ \mathrm{C}_{5} \mathrm{H}_{12}+8 \mathrm{O}_{2} \longrightarrow 6 \mathrm{H}_{2} \mathrm{O}+5 \mathrm{CO}_{2} \] how many moles of \( \mathrm{C}_{5} \mathrm{H}_{12} \) are needed to make \( 18.0 \) mol \( \mathrm{H}_{2} \mathrm{O} \) ? Show your work on your calculation sheet.