Home /
Expert Answers /
Chemistry /
f-1-0-mathrm-g-of-impure-sodium-methanoate-was-dissolved-in-water-to-make-up-a-solution-pa588
(Solved): (f) \( 1.0 \mathrm{~g} \) of impure sodium methanoate was dissolved in water to make up a solution ...
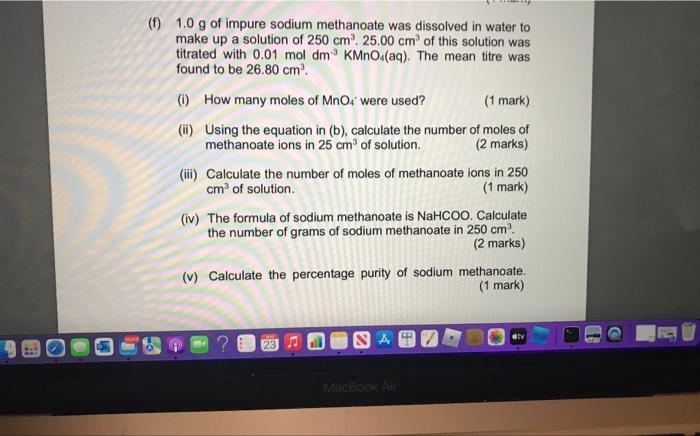

(f) \( 1.0 \mathrm{~g} \) of impure sodium methanoate was dissolved in water to make up a solution of \( 250 \mathrm{~cm}^{3} \cdot 25.00 \mathrm{~cm}^{3} \) of this solution was titrated with \( 0.01 \mathrm{~mol} \mathrm{dm}^{-3} \mathrm{KMnO}_{4}(\mathrm{aq}) \). The mean titre was found to be \( 26.80 \mathrm{~cm}^{3} \). (i) How many moles of \( \mathrm{MnO}_{4} \) - were used? (1 mark) (ii) Using the equation in (b), calculate the number of moles of methanoate ions in \( 25 \mathrm{~cm}^{3} \) of solution. (2 marks) (iii) Calculate the number of moles of methanoate ions in 250 \( \mathrm{cm}^{3} \) of solution. (1 mark) (iv) The formula of sodium methanoate is \( \mathrm{NaHCOO} \). Calculate the number of grams of sodium methanoate in \( 250 \mathrm{~cm}^{3} \). (2 marks) (v) Calculate the percentage purity of sodium methanoate. (1 mark)
22. Consider the following half equations involving the methanoate ion and the manganate(VII) ion: \[ \begin{array}{l} \mathrm{HCOO}(\mathrm{aq}) \rightarrow \mathrm{CO}_{2}(\mathrm{~g})+\mathrm{H}^{*}(\mathrm{aq})+2 \mathrm{e}^{-} \\ \mathrm{MnO}_{4}(\mathrm{aq})+5 \mathrm{e}^{-}+8 \mathrm{H}^{*}(\mathrm{aq}) \rightarrow \mathrm{Mn}^{2+}(\mathrm{aq})+4 \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \end{array} \] (a) Draw the displayed structure of the methanoate ion. \( (1 \) mark) (b) Write the balanced overall equation between the methanoate and the manganate(VII) ions. (2 marks) (c) Identify the reducing and the oxidising agent of this reaction. (2 marks) (d) This reaction is relatively slow at first. Explain why. (2 marks) (e) After a while, the reaction rate will increase. Suggest why. (1 mark) (f) \( 1.0 \mathrm{~g} \) of impure sodium methanoate was dissolved in water to make up a solution of \( 250 \mathrm{~cm}^{2}, 25.00 \mathrm{~cm}^{3} \) of this solution was titrated with \( 0.01 \mathrm{~mol} \mathrm{dm}^{-3} \mathrm{KMnO}_{4}(\mathrm{aq}) \). The mean titre was found to be \( 26.80 \mathrm{~cm}^{3} \). (i) How many moles of \( \mathrm{MnO}_{4} \) ' were used? (1 mark) (ii) Using the equation in (b), calculate the number of moles of methanoate ions in \( 25 \mathrm{~cm}^{3} \) of solution. (2 marks) (iii) Calculate the number of moles of methanoate ions in 250 \( \mathrm{cm}^{2} \) of solution. (1 mark) (iv) The formula of sodium methanoate is NaHCOO. Calculate the number of grams of sodium methanoate in \( 250 \mathrm{~cm} \) ? (2 marks) (v) Calculate the percentage purity of sodium methanoate.