Home /
Expert Answers /
Chemistry /
example-1-calculate-the-pka-value-of-the-weakly-basic-aromatic-amine-in-procaine-from-the-data-g-pa716
(Solved): - Example 1: Calculate the pKa value of the weakly basic aromatic amine in procaine from the data g ...
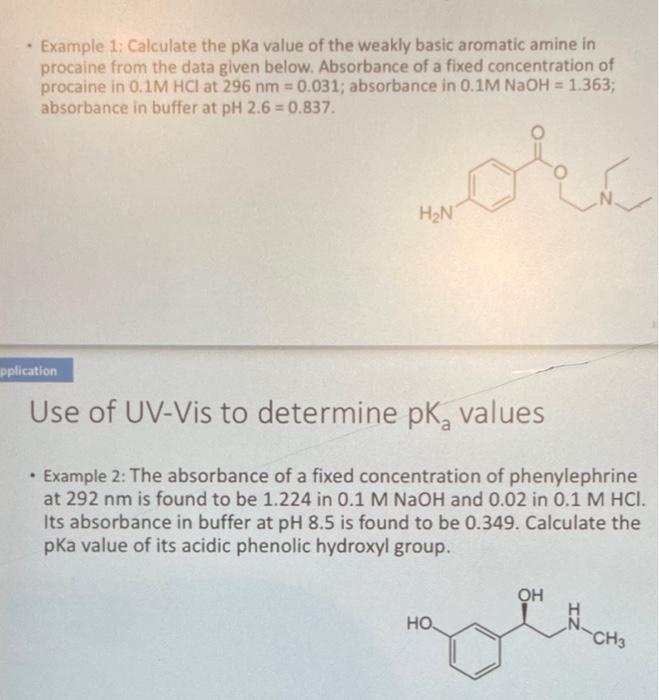
- Example 1: Calculate the pKa value of the weakly basic aromatic amine in procaine from the data given below. Absorbance of a fixed concentration of procaine in \( 0.1 \mathrm{M} \mathrm{HCl} \) at \( 296 \mathrm{~nm}=0.031 \); absorbance in \( 0.1 \mathrm{M} \mathrm{NaOH}=1.363 \); absorbance in buffer at \( \mathrm{pH} 2.6=0.837 \). Use of UV-Vis to determine \( \mathrm{pK}_{\mathrm{a}} \) values - Example 2: The absorbance of a fixed concentration of phenylephrine at \( 292 \mathrm{~nm} \) is found to be \( 1.224 \) in \( 0.1 \mathrm{M} \mathrm{NaOH} \) and \( 0.02 \) in \( 0.1 \mathrm{M} \mathrm{HCl} \). Its absorbance in buffer at \( \mathrm{pH} 8.5 \) is found to be \( 0.349 \). Calculate the pKa value of its acidic phenolic hydroxyl group.
Expert Answer
suppose that the weakly basic grammatic amine is "A" in 1m HCl "A" is completely protonated to HAA+ In 0.1 m NAOH "A" remains deprotonated as "A"