Home /
Expert Answers /
Chemical Engineering /
ethylene-is-partially-oxidized-to-produce-ethylene-oxide-eo-in-a-continuous-reactor-mathrm-c-pa515
(Solved): Ethylene is partially oxidized to produce ethylene oxide (EO) in a continuous reactor. \[ \mathrm{C ...
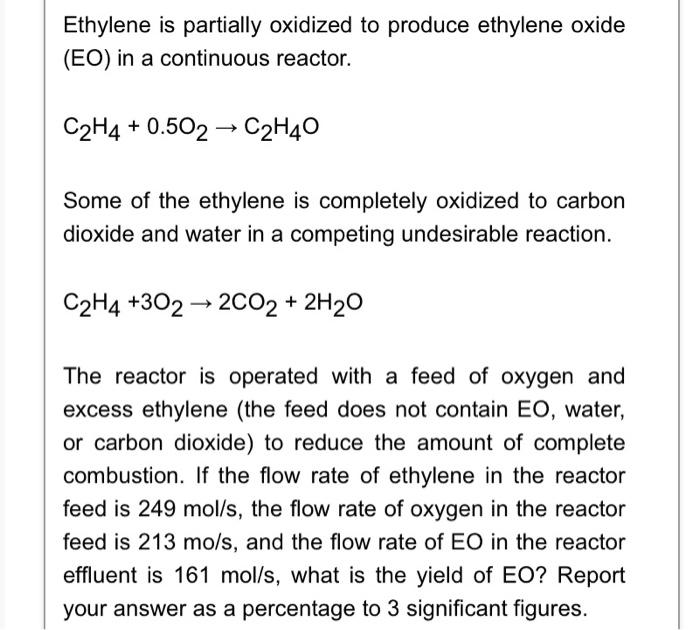
Ethylene is partially oxidized to produce ethylene oxide (EO) in a continuous reactor. \[ \mathrm{C}_{2} \mathrm{H}_{4}+0.5 \mathrm{O}_{2} \rightarrow \mathrm{C}_{2} \mathrm{H}_{4} \mathrm{O} \] Some of the ethylene is completely oxidized to carbon dioxide and water in a competing undesirable reaction. \[ \mathrm{C}_{2} \mathrm{H}_{4}+3 \mathrm{O}_{2} \rightarrow 2 \mathrm{CO}_{2}+2 \mathrm{H}_{2} \mathrm{O} \] The reactor is operated with a feed of oxygen and excess ethylene (the feed does not contain EO, water, or carbon dioxide) to reduce the amount of complete combustion. If the flow rate of ethylene in the reactor feed is \( 249 \mathrm{~mol} / \mathrm{s} \), the flow rate of oxygen in the reactor feed is \( 213 \mathrm{mo} / \mathrm{s} \), and the flow rate of EO in the reactor effluent is \( 161 \mathrm{~mol} / \mathrm{s} \), what is the yield of EO? Report your answer as a percentage to 3 significant figures.