Home /
Expert Answers /
Chemical Engineering /
ethanol-is-produced-commercially-by-the-hydration-of-ethylene-c2h4-g-h2o-v-c2h5-pa351
(Solved): Ethanol is produced commercially by the hydration of ethylene: C2H4(g)+H2O(v)C2H5 ...
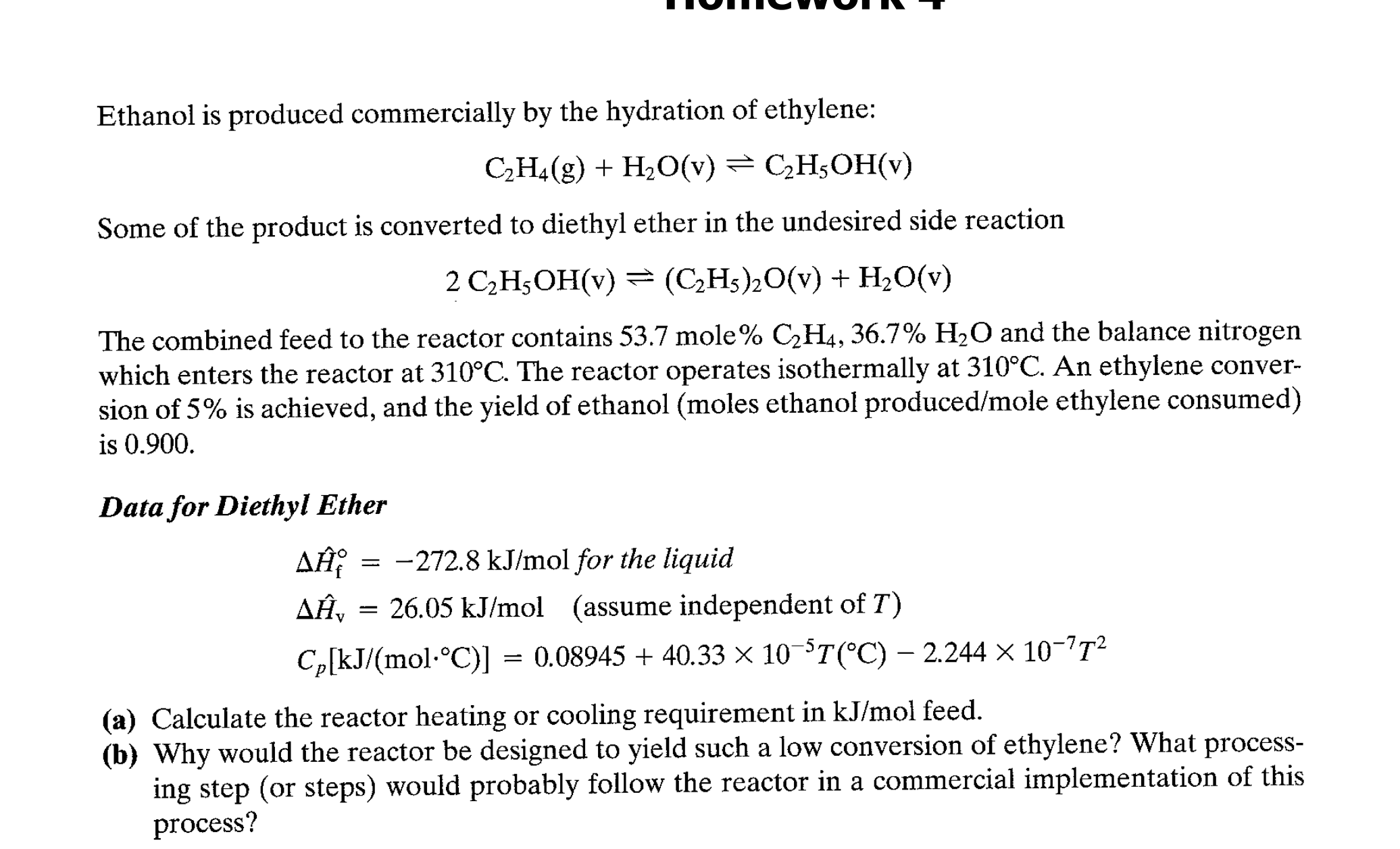
Ethanol is produced commercially by the hydration of ethylene: Some of the product is converted to diethyl ether in the undesired side reaction The combined feed to the reactor contains 53.7 mole and the balance nitrogen which enters the reactor at . The reactor operates isothermally at . An ethylene conversion of is achieved, and the yield of ethanol (moles ethanol produced/mole ethylene consumed) is 0.900 . Data for Diethyl Ether (a) Calculate the reactor heating or cooling requirement in feed. (b) Why would the reactor be designed to yield such a low conversion of ethylene? What processing step (or steps) would probably follow the reactor in a commercial implementation of this process?
Expert Answer
The primary reaction is and an undesirable sidereaction is (a) Calculate the number of moles of , in the exit product, . Recall from the problem statement that the conversion of ethylene is 5%, so 95% of the amount in the feed exits in the product. Use a basis of 1 mol feed