Home /
Expert Answers /
Chemistry /
enter-the-coefficients-directly-from-the-balanced-chemical-equation-do-not-reduce-to-lowest-terms-pa116
(Solved): Enter the coefficients directly from the balanced chemical equation. Do not reduce to lowest terms. ...
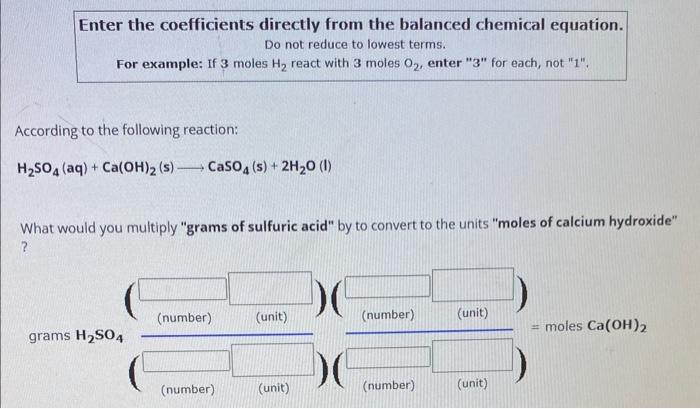
Enter the coefficients directly from the balanced chemical equation. Do not reduce to lowest terms. For example: If 3 moles \( \mathrm{H}_{2} \) react with 3 moles \( \mathrm{O}_{2} \), enter "3" for each, not "1". According to the following reaction: \[ \mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq})+\mathrm{Ca}(\mathrm{OH})_{2}(\mathrm{~s}) \longrightarrow \mathrm{CaSO}_{4}(\mathrm{~s})+2 \mathrm{H}_{2} \mathrm{O} \text { (I) } \] What would you multiply "grams of sulfuric acid" by to convert to the units "moles of calcium hydroxide" ?
Expert Answer
The problem can be solved by dimensional analysis for which we