Home /
Expert Answers /
Chemistry /
edta-is-a-hexaprotic-system-with-the-pka-values-pka1-0-00-pka2-1-50-pka3-2-00-pka4-pa438
(Solved): EDTA is a hexaprotic system with the pKa values: pKa1=0.00,pKa2=1.50,pKa3=2.00,pKa4= ...
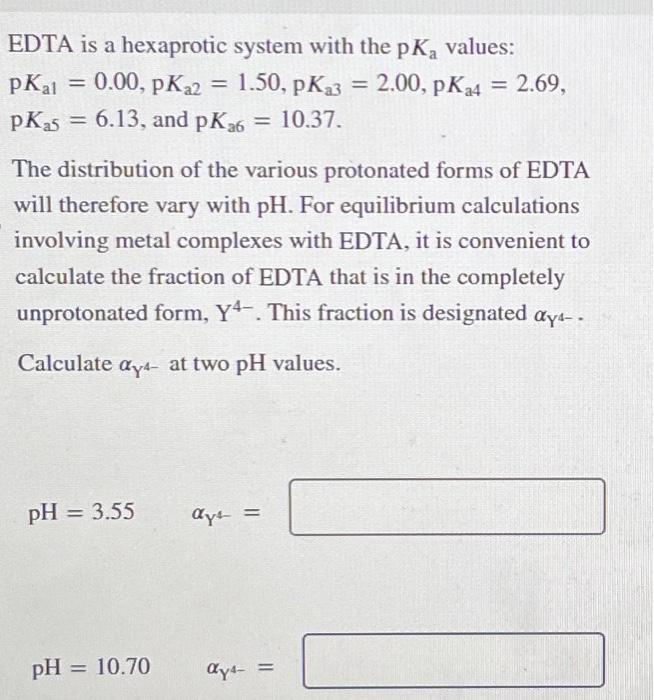
EDTA is a hexaprotic system with the values: The distribution of the various protonated forms of EDTA will therefore vary with . For equilibrium calculations involving metal complexes with EDTA, it is convenient to calculate the fraction of EDTA that is in the completely unprotonated form, . This fraction is designated . Calculate at two values.