Home /
Expert Answers /
Chemistry /
e-complete-the-reaction-table-shown-below-f-indicate-the-column-corresponding-to-the-limiting-rea-pa724
(Solved): e. Complete the reaction table shown below f. Indicate the column corresponding to the limiting rea ...
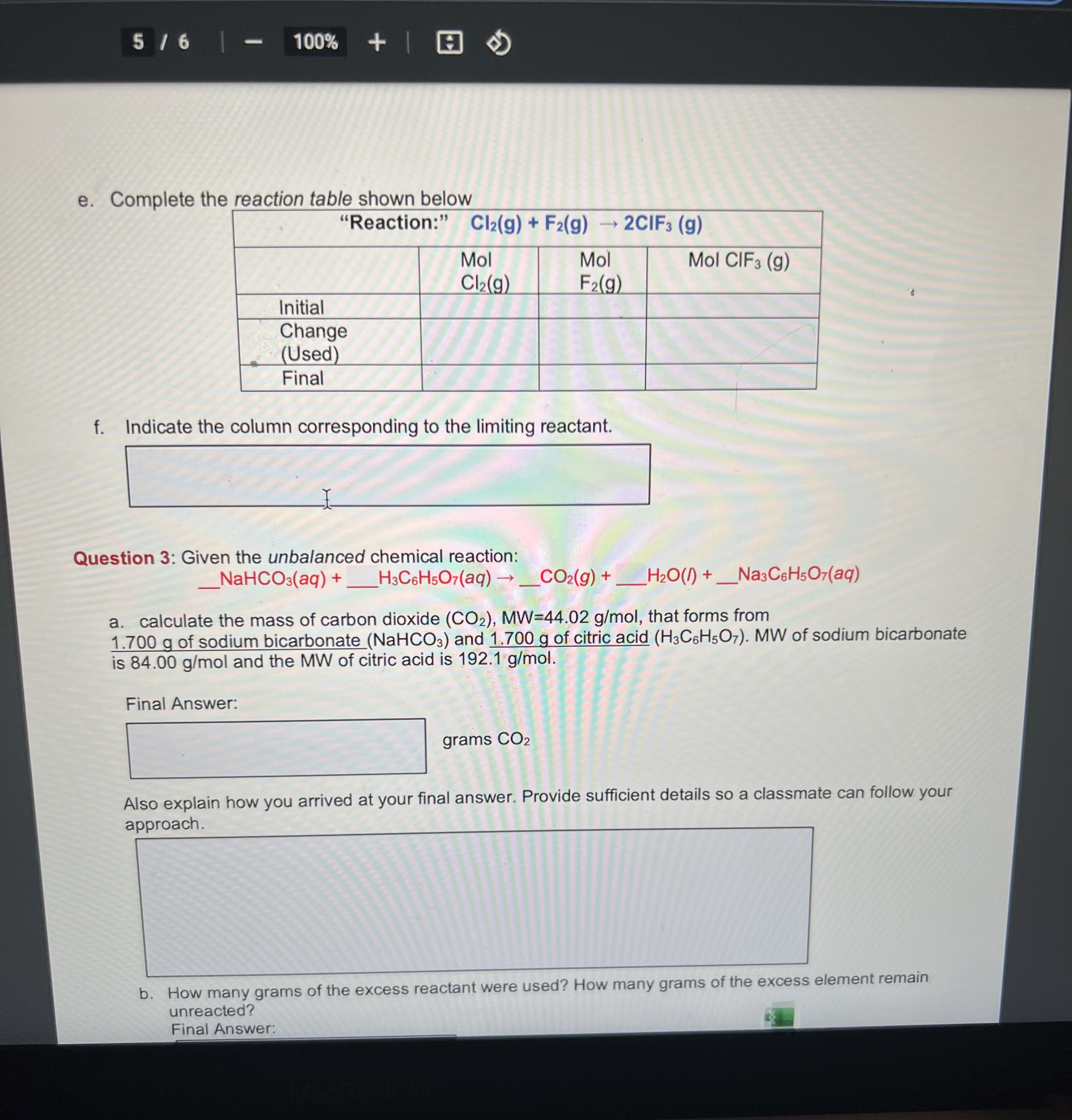
e. Complete the reaction table shown below f. Indicate the column corresponding to the limiting reactant. Question 3: Given the unbalanced chemical reaction: a. calculate the mass of carbon dioxide , that forms from of sodium bicarbonate and of citric acid . MW of sodium bicarbonate is and the MW of citric acid is . Final Answer: grams Also explain how you arrived at your final answer. Provide sufficient details so a classmate can follow your aporoach. b. How many grams of the excess reactant were used? How many grams of the excess element remain unreacted? Final Answer: