Home /
Expert Answers /
Chemistry /
draw-the-major-product-of-this-reaction-include-stereochemistry-if-applicable-ignore-byproducts-pa894
(Solved): Draw the major product of this reaction. Include stereochemistry if applicable. Ignore byproducts. ...
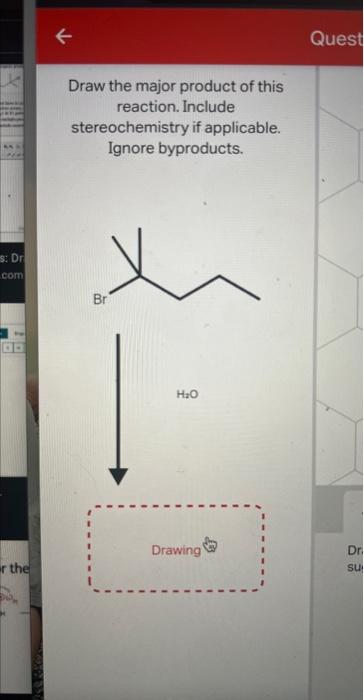

Draw the major product of this reaction. Include stereochemistry if applicable. Ignore byproducts.
Draw the major organic product of this E1 elimination reaction. Ignore byproducts.
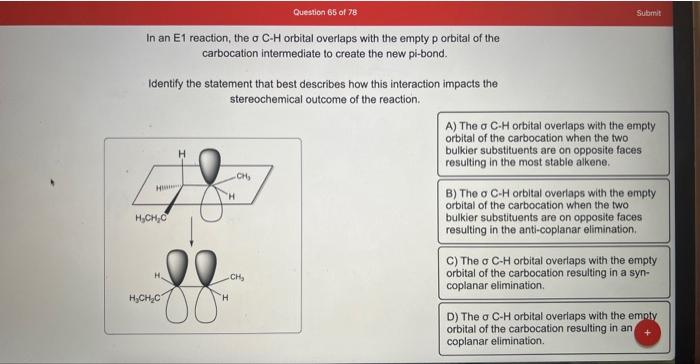
In an \( \mathrm{E} 1 \) reaction, the \( \sigma \mathrm{C}-\mathrm{H} \) orbital overlaps with the empty p orbital of the carbocation intermediate to create the new pi-bond. Identify the statement that best describes how this interaction impacts the stereochemical outcome of the reaction. A) The o C.H orbital overlaps with the empty orbital of the carbocation when the two bulkier substituents are on opposite faces resulting in the most stable alkene. B) The o C-H orbltal overlaps with the empty orbital of the carbocation when the two bulkier substituents are on opposite faces resulting in the anti-coplanar elimination. C) The o C-H orbital overlaps with the empty orbital of the carbocation resulting in a syncoplanar elimination. D) The o C-H orbital overlaps with the empty orbital of the carbocation resulting in an coplanar elimination.
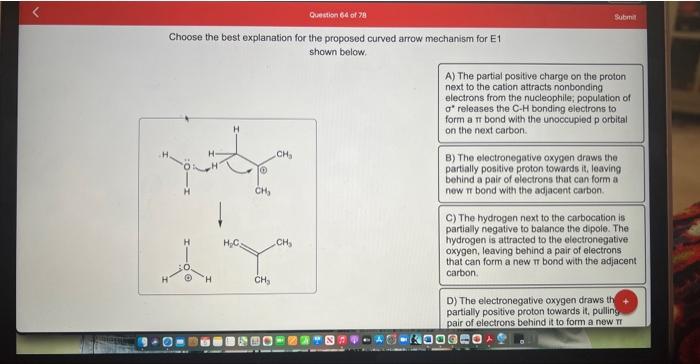
Choose the best explanation for the proposed curved arrow mechanism for E1 shown below. A) The partial positive charge on the proton next to the cation attracts nonbonding electrons from the nucleophile; population of \( \sigma^{*} \) releases the C-H bonding electrons to form a \( \pi \) bond with the unoccupied p orbital on the next carbon. B) The electronegative oxygen draws the partially positive proton towards it, leaving behind a pair of electrons that can form a new \( \pi \) bond with the adjacent carbon. C) The hydrogen next to the carbocation is partially negative to balance the di pole. The hydrogen is attracted to the electronegative oxygen, leaving behind a pair of electrons that can form a new \( \pi \) bond with the adjacent carbon. D) The electronegative oxygen draws th \( + \) partially positive proton towards it, pulling pair of electrons behind it to form a new \( \pi \)
Expert Answer
When reaction of alkyl halide takes place with water then O