Home /
Expert Answers /
Chemistry /
draw-all-resonance-structures-for-the-acetate-ion-mathrm-ch-3-mathrm-coo-explic-pa551
(Solved): Draw all resonance structures for the acetate ion, \( \mathrm{CH}_{3} \mathrm{COO}^{-} \). - Explic ...
Draw all resonance structures for the acetate ion, \( \mathrm{CH}_{3} \mathrm{COO}^{-} \). - Explicitly draw all \( \mathrm{H} \) atoms. - Include all valence lone pairs in your answer. - Do not include overall lon charges or formal charges in your drawing. - Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. - Draw one structure per sketcher. Add additional sketchers by selecting \( \leftrightarrow \) in the drop-down menu
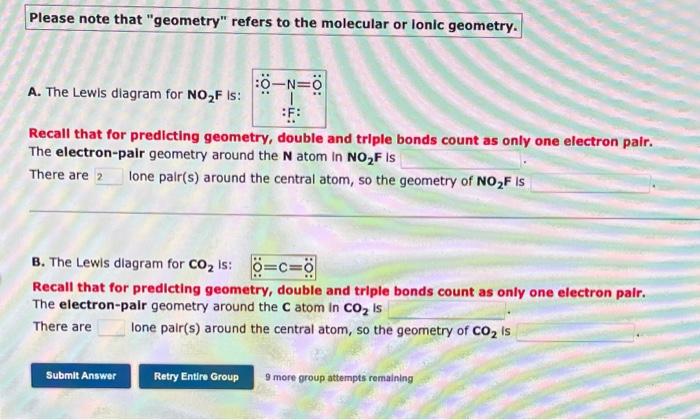
Please note that "geometry" refers to the molecular or lonic geometry. A. The Lewis diagram for \( \mathrm{NO}_{2} \mathrm{~F} \) is: Recall that for predicting geometry, double and triple bonds count as only one electron pair. The electron-pair geometry around the \( \mathbf{N} \) atom in \( \mathrm{NO}_{2} \mathrm{~F} \) is There are Ione pair(s) around the central atom, so the geometry of \( \mathrm{NO}_{2} \mathrm{~F} \) is B. The Lewis diagram for \( \mathrm{CO}_{2} \) is: Recall that for predicting geometry, double and triple bonds count as only one electron pair. The electron-palr geometry around the \( \mathrm{C} \) atom in \( \mathrm{CO}_{2} \) is There are Ione pair(s) around the central atom, so the geometry of \( \mathrm{CO}_{2} \) is
Draw a Lewis structure for \( \mathrm{SiCl}_{4} \) in which the central SI atom obeys the octet rule, and answer the following questions based on your drawing. The number of unshared pairs (lone pairs) on the central SI atom Is: The central Si atom forms single bonds. The central Si atom forms double bonds. 2 more groug attempte remaining
Draw a Lewls structure for \( \mathbf{S F}_{2} \) In which the central \( \mathbf{S} \) atom obeys the octet rule, and answer the following questions based on your drawin The number of unshared pairs (lone pairs) on the central \( \mathbf{S} \) atom is: The central \( \mathbf{S} \) atom forms single bonds. The central \( \mathbf{S} \) atom forms double bonds. D mote group attempts romeining
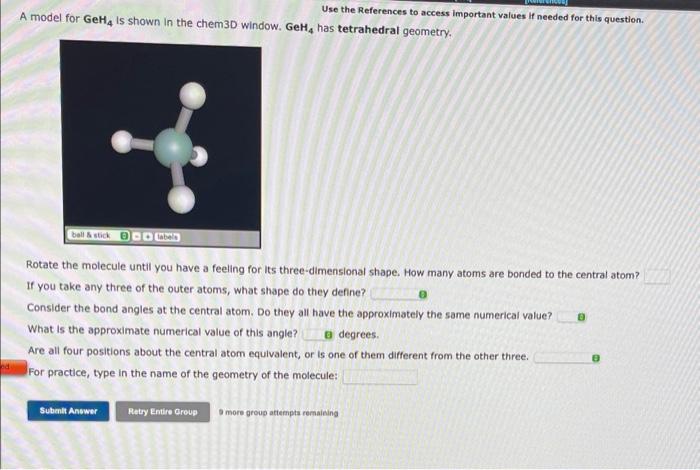
Use the References to access important values if needed for this question. A model for \( \mathrm{GeH}_{4} \) Is shown in the chem3D window. \( \mathrm{GeH}_{4} \) has tetrahedral geometry. Rotate the molecule until you have a feeling for its three-dimensional shape. How many atoms are bonded to the central atom? If you take any three of the outer atoms, what shape do they define? Consider the bond angles at the central atom. Do they all have the approximately the same numerical value? What is the approximate numerical value of this angle? degrees. Are all four positions about the central atom equivalent, or is one of them different from the other three. For practice, type in the name of the geometry of the molecule: 3 more grous atternpta remaining
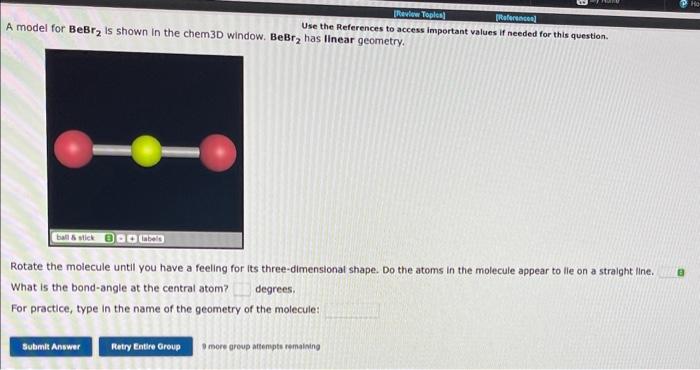
Use the References to access important values if needed for this question. A model for \( \mathrm{BeBr}_{2} \) is shown in the chem3D window. \( \mathrm{BeBr}_{2} \) has linear geometry. Rotate the molecule until you have a feeling for its three-dimensional shape. Do the atoms in the molecule appear to lle on a straight line. What is the bond-angle at the central atom? degrees. For practice, type in the name of the geometry of the molecule: B more group attempts remaining
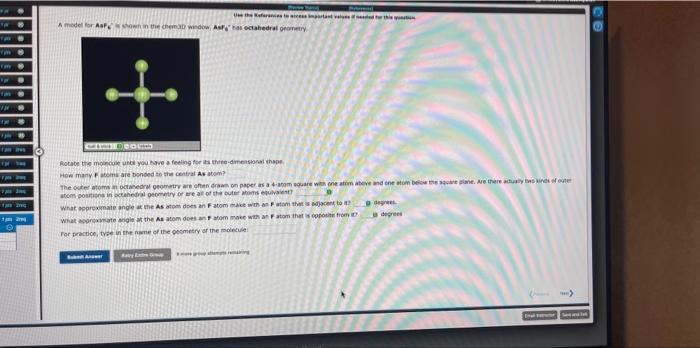
fotate the moscus urtil you kave a feelng for ita thete-dmetstorai thope Hen mary \( f \) atoint are bonced to the central As atem? What opprexmate ande at the As atem dset an \( \mathrm{F} \) atom mace with an \( \mathrm{F} \) atem thet as adjacent to at degreet. 3 aegret For practice, tree in the name of the pesmery of the moineule:
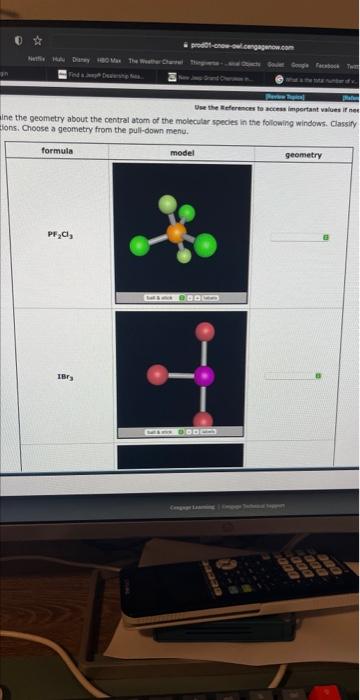
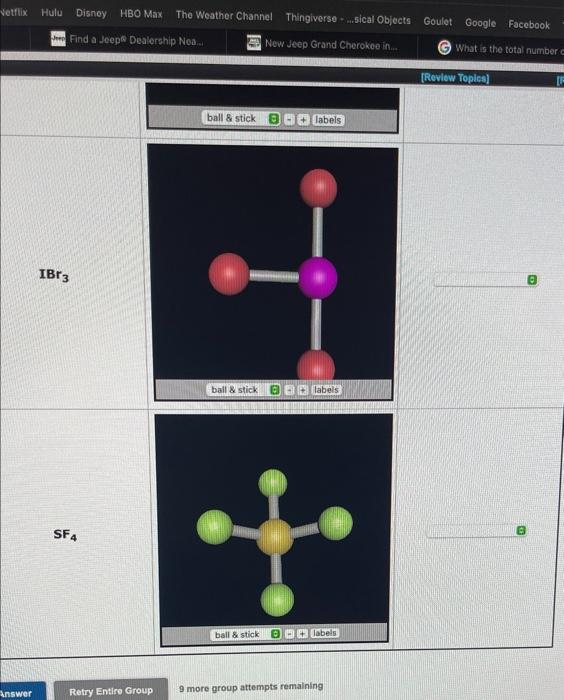
Wee the Meferences to hcteas impertant values if net Ine the geometry about the centrel atom of the molecular species in the following windows. Classify fions. Choose a geometry from the pult-down menu.
9 more group attempts remalning