Home /
Expert Answers /
Chemistry /
determine-the-mass-in-that-are-produced-by-the-complete-reaction-of-nonane-according-to-the-follo-pa982
(Solved): Determine the mass in that are produced by the complete reaction of (nonane) according to the follo ...
![according to the following reaction:
\[
\mathrm{H}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow
\]
STARTING AMOUN](https://media.cheggcdn.com/study/82c/82c1608e-0fd1-432d-91c6-99e2d9d7be21/image)

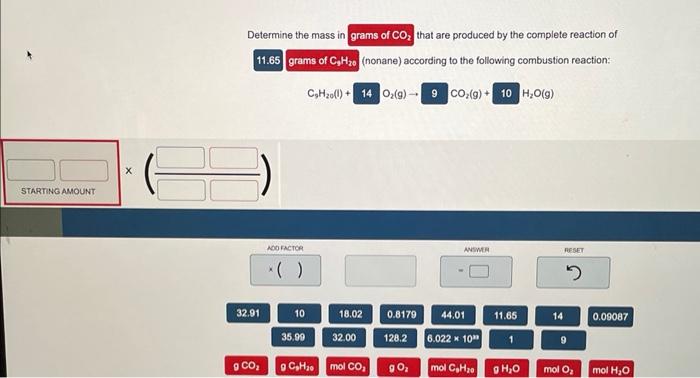
Determine the mass in that are produced by the complete reaction of (nonane) according to the following combustion reaction: \( \mathrm{C}_{9} \mathrm{H}_{2} \mathrm{O}(\mathrm{l})+\mathrm{O}_{2}(g) \rightarrow \quad \mathrm{CO}_{2}(g)+\mathrm{H}_{2} \mathrm{O}(g) \)
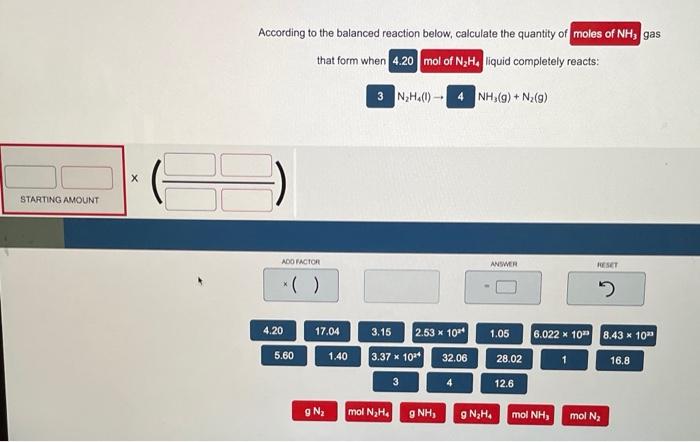
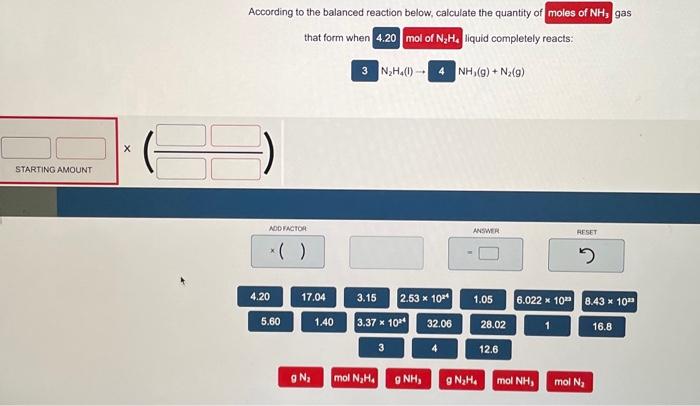
According to the balanced reaction below, calculate the quantity of gas that form when liquid completely reacts: \( \mathrm{N}_{2} \mathrm{H}_{4}(\mathrm{l}) \rightarrow \quad \mathrm{NH}_{3}(\mathrm{~g})+\mathrm{N}_{2}(\mathrm{~g}) \)
According to the balanced reaction below, calculate the quantity of gas that form when liquid completely reacts: \[ \mathrm{N}_{2} \mathrm{H}_{4}(\mathrm{l}) \rightarrow \quad \mathrm{NH}_{3}(\mathrm{~g})+\mathrm{N}_{2}(\mathrm{~g}) \]
according to the following reaction: \[ \mathrm{H}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow \] STARTING AMOUNT
How many moles of \( \mathrm{Al} \) are necessary to form \( 38.6 \mathrm{~g} \) of \( \mathrm{AlBr}_{3} \) from this reaction: \[ 2 \mathrm{Al}(\mathrm{s})+3 \mathrm{Br}_{2}(\mathrm{I}) \rightarrow 2 \mathrm{AlBr}_{3}(\mathrm{~s}) ? \]