Home /
Expert Answers /
Chemistry /
determine-the-equilibrium-constant-for-the-following-reaction-at-298-k-cl-g-o3-g-clo-g-o-pa524
(Solved): Determine the equilibrium constant for the following reaction at 298 K. Cl(g) + O3(g) ClO(g) + O ...
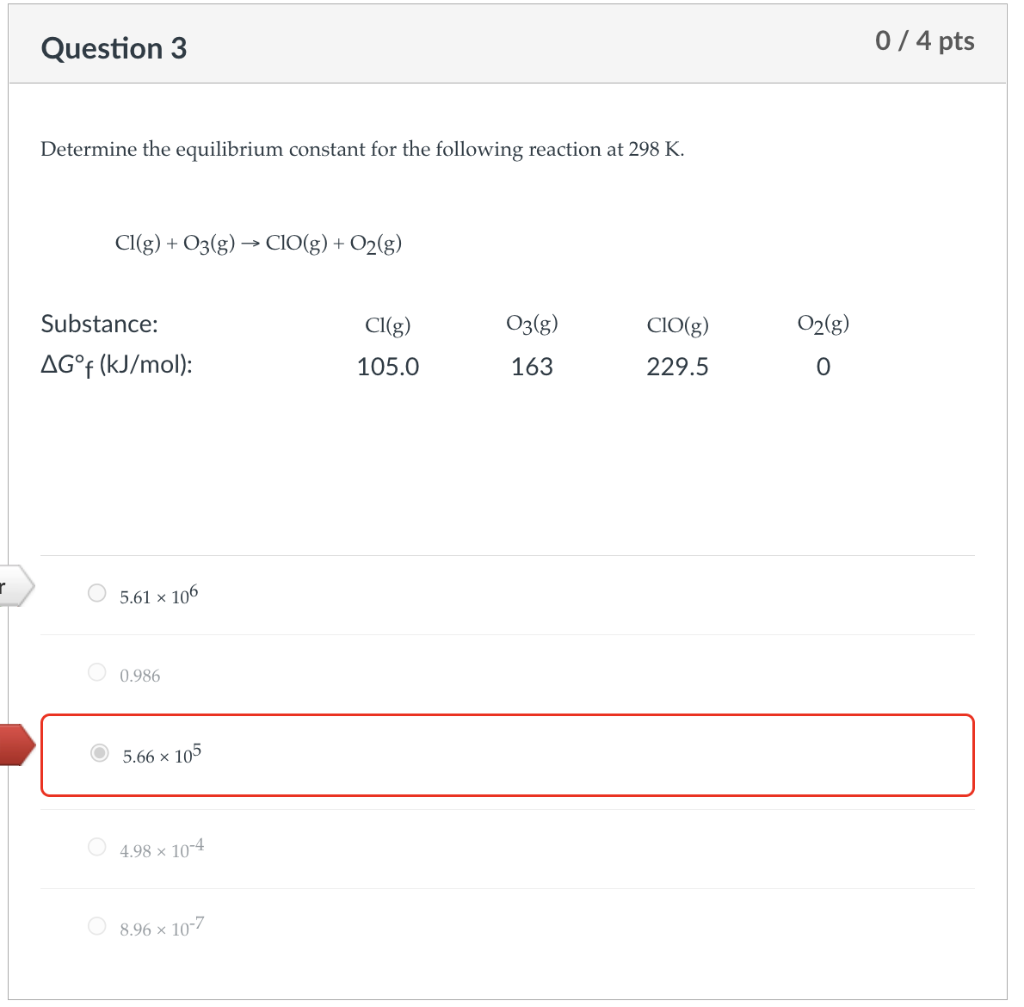
Determine the equilibrium constant for the following reaction at \( 298 \mathrm{~K} \). \[ \mathrm{Cl}(\mathrm{g})+\mathrm{O}_{3}(\mathrm{~g}) \rightarrow \mathrm{ClO}(\mathrm{g})+\mathrm{O}_{2}(\mathrm{~g}) \] \[ 5.61 \times 10^{6} \] \[ 0.986 \] \[ 5.66 \times 10^{5} \] \[ 4.98 \times 10^{-4} \] \[ 8.96 \times 10^{-7} \]