Home /
Expert Answers /
Chemistry /
data-table-a-solubility-and-conductivity-soluble-substance-insoluble-soluble-1-vinegar-solution-c-pa115
(Solved): Data Table A: Solubility and Conductivity Soluble Substance Insoluble soluble 1. Vinegar solution, C ...
Data Table A: Solubility and Conductivity Soluble Substance Insoluble soluble 1. Vinegar solution, CH3COOH 2.0.1 M HCI 3. Vegetable Oil, 4. Cholesterol, C27H46O 5. Sodium Hydroxide, NaOH 6. Glucose, C6H12O6 7. Sodium Chloride, NaCl Data Table B: pH and [H+] pH Substance 1. Vinegar 2. Hydrochloric acid 3. Vegetable oil 4. Cholesterol 5. Sodium hydroxide 6. Glucose 7. Sodium Chloride 29 2.9 1.1 7.4 6.7 10.4 7.0 7.1 Soluble Not soluble Not soluble soluble Soluble soluble Conductivity weak strong Non conductor Non conductor Strong weak strong Calculated [H+] Concentration. 1.26×10 Electrolyte Classification 10-PH
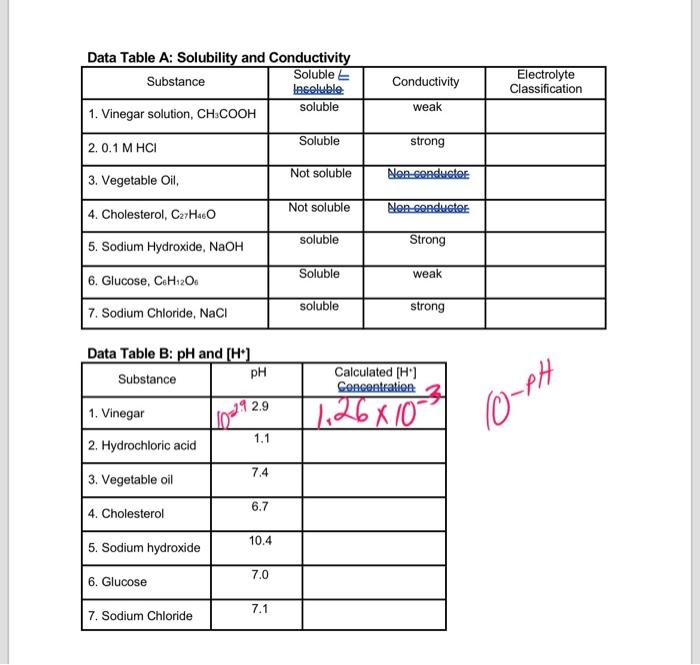
Rada Tabla D. WU and rL*1
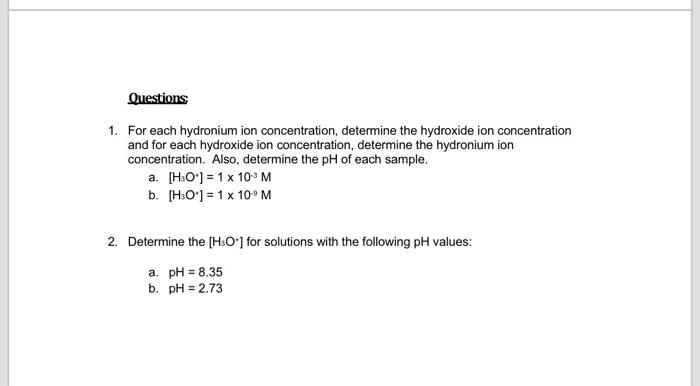
1. For each hydronium ion concentration, determine the hydroxide ion concentration and for each hydroxide ion concentration, determine the hydronium ion concentration. Also, determine the of each sample. a. b. 2. Determine the for solutions with the following values: a. b.
Expert Answer
Table A:Electrolyte classification-1) Vinegar: weak electrolyte Since it is partially dissociated in the aqueous solution.2) 0.1 M HCl: Strong electrolyteSince it dissociates completely in aqueous solution.3) Vegetable oil: Not act as an electrolyte4) Cholesterol: Not act as an electrolyte5) Sodium hydroxide: Strong electrolyteSince it dissociates completely in aqueous solution.6) Glucose: weak electrolyte Since it is partially dissociated in the aqueous solution.7) Sodium chloride: strong electrolyte Since it dissociates completely in aqueous solution.