Home /
Expert Answers /
Chemistry /
correct-correct-answer-is-shown-your-answer-11-80351-mathrm-mol-mathrm-l-1-was-eith-pa541
(Solved): Correct Correct answer is shown. Your answer \( 11.80351 \mathrm{~mol} \mathrm{~L}^{-1} \) was eith ...
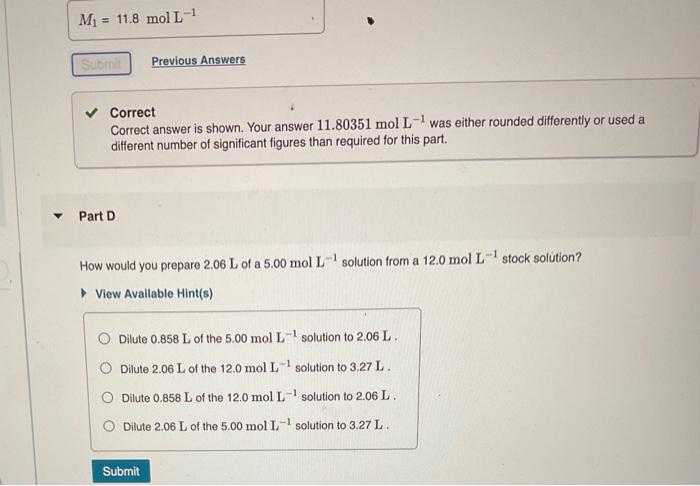
Correct Correct answer is shown. Your answer \( 11.80351 \mathrm{~mol} \mathrm{~L}^{-1} \) was either rounded differently or used a different number of significant figures than required for this part. Part D How would you prepare \( 2.06 \mathrm{~L} \) of a \( 5.00 \mathrm{~mol} \mathrm{~L}^{-1} \) solution from a \( 12.0 \mathrm{~mol} \mathrm{~L}^{-1} \) stock solution? View Available Hint(s) Dilute \( 0.858 \mathrm{~L} \) of the \( 5.00 \mathrm{~mol} \mathrm{~L}^{-1} \) solution to \( 2.06 \mathrm{~L} \). Dilute \( 2.06 \mathrm{~L} \) of the \( 12.0 \mathrm{~mol} \mathrm{~L}^{-1} \) solution to \( 3.27 \mathrm{~L} \). Dilute \( 0.858 \mathrm{~L} \) of the \( 12.0 \mathrm{~mol} \mathrm{~L}^{-1} \) solution to \( 2.06 \mathrm{~L} \). Dilute \( 2.06 \mathrm{~L} \) of the \( 5.00 \mathrm{~mol} \mathrm{~L}^{-1} \) solution to \( 3.27 \mathrm{~L} \).
Expert Answer
To solve this question we need to use the concept of dilution . When diluted the no of moles of the solute never actually changes which