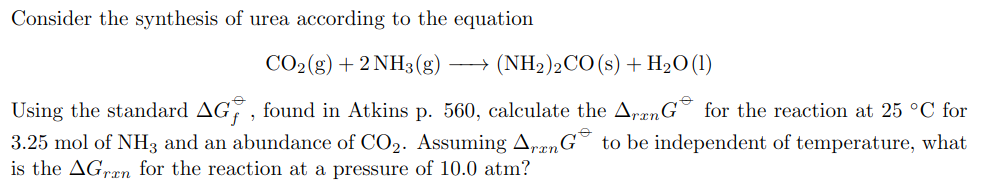Home /
Expert Answers /
Chemistry /
consider-the-synthesis-of-urea-according-to-the-equation-mathrm-co-2-mathrm-g-2-mathrm-pa357
(Solved): Consider the synthesis of urea according to the equation \[ \mathrm{CO}_{2}(\mathrm{~g})+2 \mathrm ...
Consider the synthesis of urea according to the equation \[ \mathrm{CO}_{2}(\mathrm{~g})+2 \mathrm{NH}_{3}(\mathrm{~g}) \longrightarrow\left(\mathrm{NH}_{2}\right)_{2} \mathrm{CO}(\mathrm{s})+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \] Using the standard \( \Delta G_{f}^{\ominus} \), found in Atkins p. 560 , calculate the \( \Delta_{r x n} G^{\ominus} \) for the reaction at \( 25^{\circ} \mathrm{C} \) for \( 3.25 \mathrm{~mol} \) of \( \mathrm{NH}_{3} \) and an abundance of \( \mathrm{CO}_{2} \). Assuming \( \Delta_{r x n} G^{\ominus} \) to be independent of temperature, what is the \( \Delta G_{r x n} \) for the reaction at a pressure of \( 10.0 \mathrm{~atm} \) ?