Home /
Expert Answers /
Chemistry /
consider-the-reaction-between-phosphorus-and-hydrogen-shown-below-p4-s-6h2-g-gt-4-ph3-g-pa-pa512
(Solved): consider the reaction between phosphorus and hydrogen shown below:P4 (s) + 6H2 (g) -> 4 PH3 (g)Pa ...
consider the reaction between phosphorus and hydrogen shown below:
P4 (s) + 6H2 (g) -> 4 PH3 (g)
Part 1:
if 1.00 mole of P4 (s) is reacted completely, how many grams of product will be produced? Express your answer in units of grams using at least three significant figures.
Part 2:
how many grams of H2 (g) will be consumed when 1.00 mol of P4 (s) undergoes this reaction? Express your answer in units of grams using at least three significant figures.
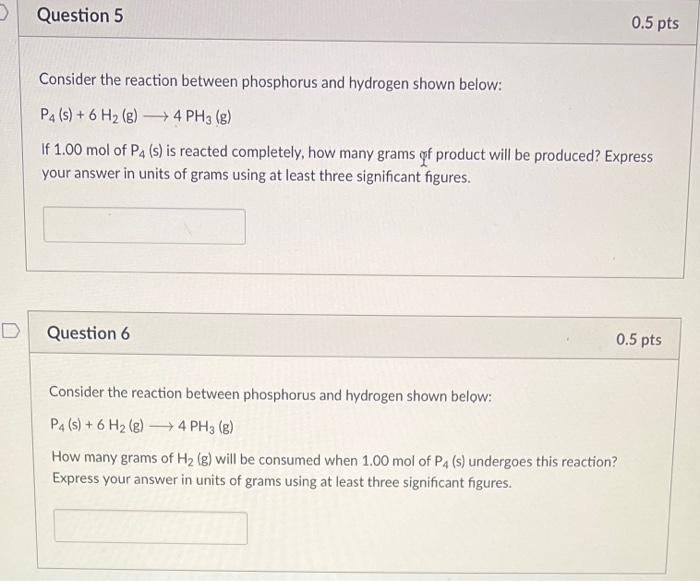
Consider the reaction between phosphorus and hydrogen shown below: If of is reacted completely, how many grams of product will be produced? Express your answer in units of grams using at least three significant figures. Question 6 Consider the reaction between phosphorus and hydrogen shown below: How many grams of will be consumed when 1.00 mol of undergoes this reaction? Express your answer in units of grams using at least three significant figures.