Home /
Expert Answers /
Chemistry /
consider-the-reaction-between-hf-and-sbf5-to-form-the-strong-acid-hsbf6-f-1st-attempt-fee-pa617
(Solved): Consider the reaction between HF and SbF5 to form the strong acid HSbF6. *.- * :F: 1st attempt Fee ...
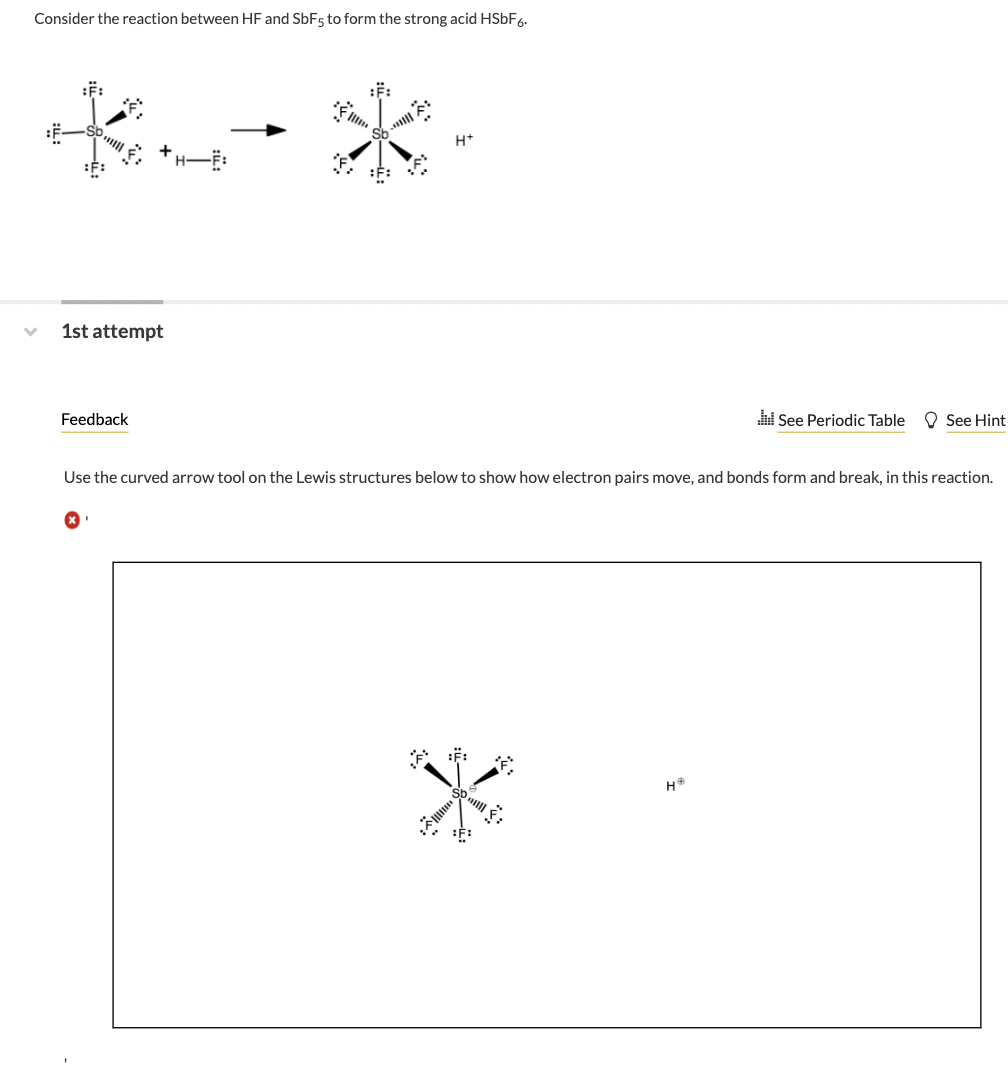
Consider the reaction between HF and SbF5 to form the strong acid HSbF6. *.- * :F: 1st attempt Feedback x H+ Use the curved arrow tool on the Lewis structures below to show how electron pairs move, and bonds form and break, in this reaction. * Sb will :^: See Periodic Table See Hint H?