Home /
Expert Answers /
Chemistry /
consider-the-reaction-and-potential-energy-diagram-below-frac-mathrm-a-mathrm-b-text-r-pa483
(Solved): Consider the reaction and potential energy diagram below: \[ \frac{\mathrm{A}+\mathrm{B}}{\text { R ...
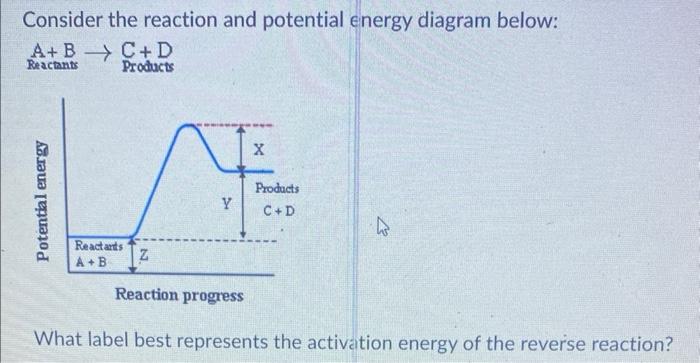
![What label best represents the activation energy of the revel
\[
X+Y
\]](https://media.cheggcdn.com/study/3c8/3c821176-6804-4860-b8d1-8b9cb679ec24/image)
![Consider the following hypothetical reaction:
\[
a A+b B \rightarrow x X+y Y
\]
Which equation correctly represents the equat](https://media.cheggcdn.com/study/a3c/a3c57591-927f-4f12-8617-26bfa0e08396/image)
Consider the reaction and potential energy diagram below: \[ \frac{\mathrm{A}+\mathrm{B}}{\text { Reactants }} \rightarrow \text { Products } \] What label best represents the activation energy of the reverse reaction?
What label best represents the activation energy of the revel \[ X+Y \]
Consider the following hypothetical reaction: \[ a A+b B \rightarrow x X+y Y \] Which equation correctly represents the equation to determine the value of the standard enthalpy of the reaction? \[ \begin{array}{l} {\left[x \Delta H^{\circ}(X)+y \Delta H^{\circ}(Y)\right]-\left[a \Delta H^{\circ}(A)+b \Delta H^{\circ}(B)\right]} \\ {\left[x \Delta H^{\circ}(X)-y \Delta H^{\circ}(Y)\right]-\left[a \Delta H^{\circ}(A)-b \Delta H^{\circ}(B)\right]} \\ {\left[x \Delta H^{\circ}(X)+y \Delta H^{\circ}(Y)\right]+\left[a \Delta H^{\circ}(A)+b \Delta H^{\circ}(B)\right]} \\ {\left[a \Delta H^{\circ}(A)-b \Delta H^{\circ}(B)\right]-\left[x \Delta H^{\circ}(X)+y \Delta H^{\circ}(Y)\right]} \end{array} \]
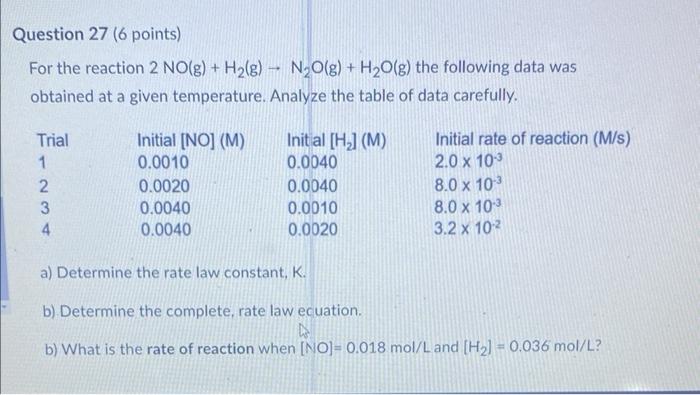
For the reaction \( 2 \mathrm{NO}(\mathrm{g})+\mathrm{H}_{2}(\mathrm{~g}) \rightarrow \mathrm{N}_{2} \mathrm{O}(\mathrm{g})+\mathrm{H}_{2} \mathrm{O}(\mathrm{g}) \) the following data was obtained at a given temperature. Analyze the table of data carefully. (M/s) a) Determine the rate law constant, \( K \). b) Determine the complete, rate law ecuation. b) What is the rate of reaction when \( [\mathrm{NO}]=0.018 \mathrm{~mol} / \mathrm{L} \) and \( \left[\mathrm{H}_{2}\right]=0.036 \mathrm{~mol} / \mathrm{L} \) ?