Home /
Expert Answers /
Chemistry /
consider-the-insoluble-compound-aluminum-phosphate-aipo4-the-aluminum-ion-also-forms-a-complex-pa885
(Solved): Consider the insoluble compound aluminum phosphate, AIPO4. The aluminum ion also forms a complex ...
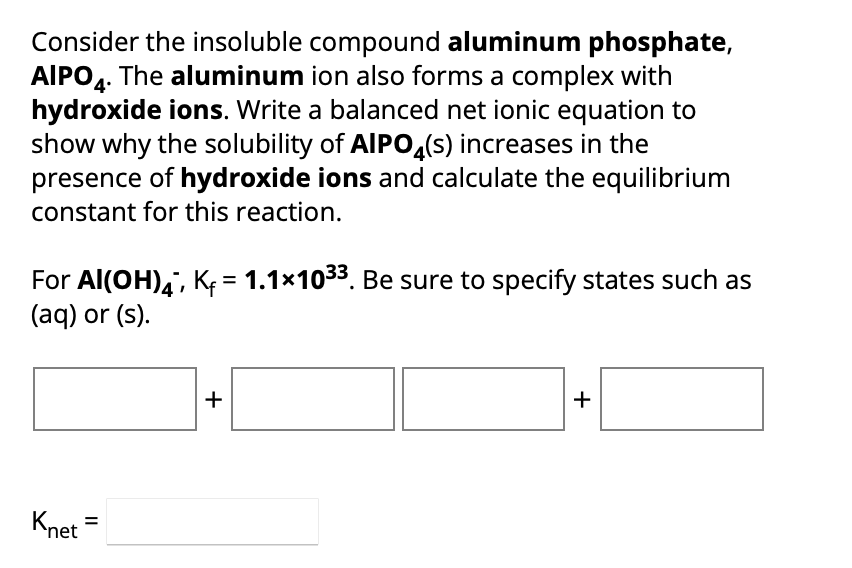
Consider the insoluble compound aluminum phosphate, . The aluminum ion also forms a complex with hydroxide ions. Write a balanced net ionic equation to show why the solubility of increases in the presence of hydroxide ions and calculate the equilibrium constant for this reaction. For . Be sure to specify states such as (aq) or (s).
Expert Answer
GivenKsp for AlPO4= 1.3 x 10-20Kf for = 1.1 x 1033i) Solubility of AlPO4 increases in the presence...