Home /
Expert Answers /
Chemistry /
consider-the-following-reaction-at-equilibrium-what-effect-will-increasing-the-temperature-have-o-pa338
(Solved): Consider the following reaction at equilibrium. What effect will increasing the temperature have o ...
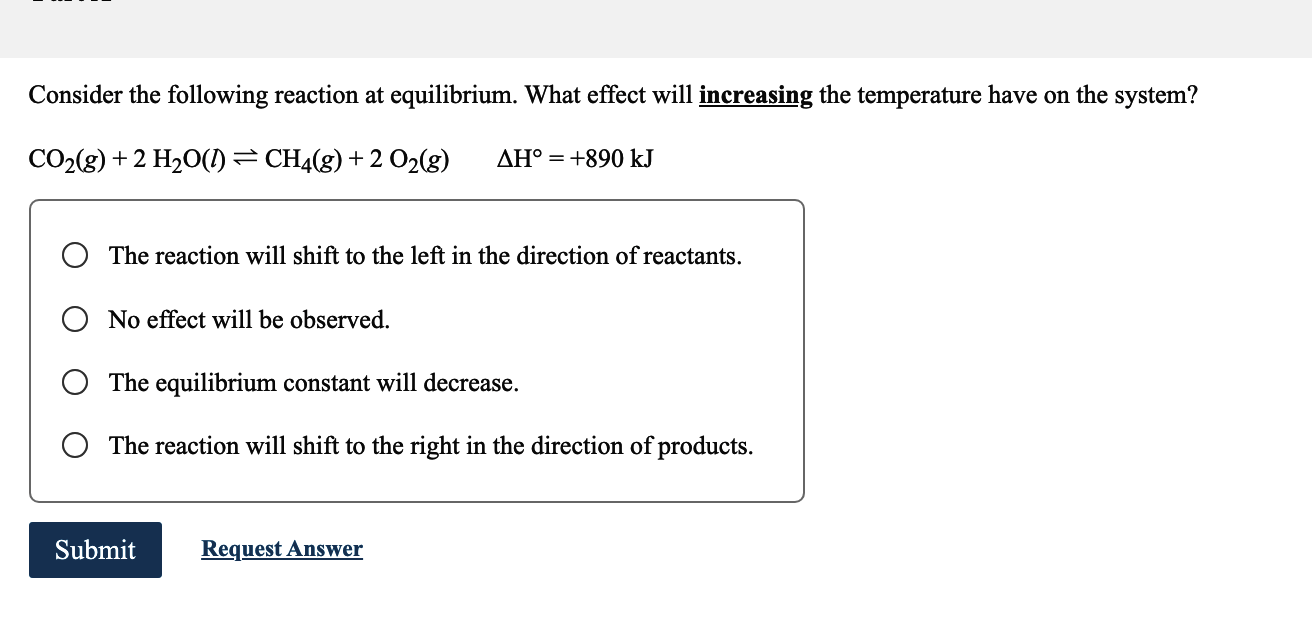
Consider the following reaction at equilibrium. What effect will increasing the temperature have on the system? The reaction will shift to the left in the direction of reactants. No effect will be observed. The equilibrium constant will decrease. The reaction will shift to the right in the direction of products.
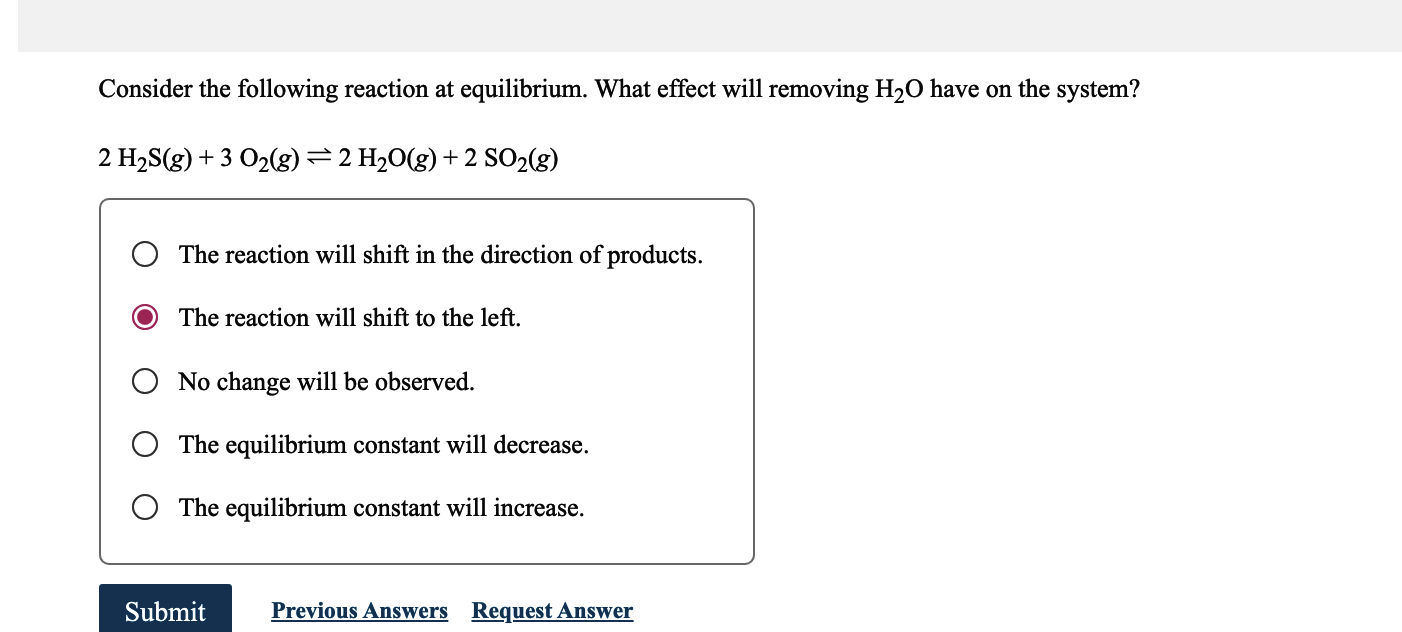
Consider the following reaction at equilibrium. What effect will removing have on the system? The reaction will shift in the direction of products. The reaction will shift to the left. No change will be observed. The equilibrium constant will decrease. The equilibrium constant will increase.
Expert Answer
In the given reaction, increasing the temperature will have the effect of shifting the equilibrium t...