Home /
Expert Answers /
Chemistry /
consider-the-following-molecular-orbital-diagram-for-the-diatomic-systems-mathrm-o-2-mathrm-pa232
(Solved): Consider the following molecular orbital diagram for the diatomic systems \( \mathrm{O}_{2}-\mathrm ...
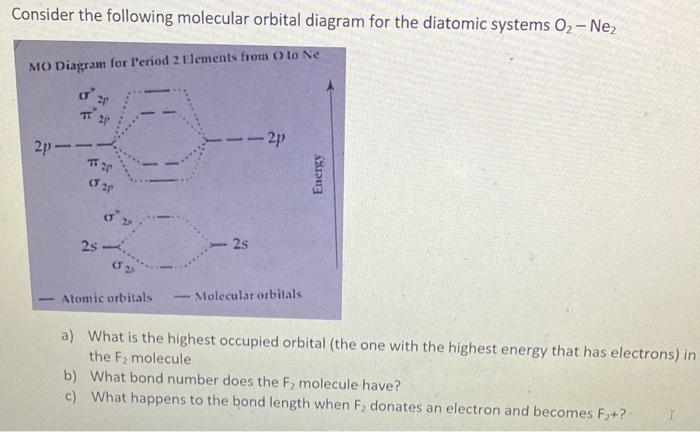
Consider the following molecular orbital diagram for the diatomic systems \( \mathrm{O}_{2}-\mathrm{Ne}_{2} \) a) What is the highest occupied orbital (the one with the highest energy that has electrons) in the \( \mathrm{F}_{2} \) molecule b) What bond number does the \( \mathrm{F}_{2} \) molecule have? c) What happens to the bond length when \( \mathrm{F}_{2} \) donates an electron and becomes \( \mathrm{F}_{2}+ \) ?