Home /
Expert Answers /
Chemistry /
consider-the-following-mechanism-for-the-oxidation-of-bromide-ions-by-hydrogen-peroxide-in-aqueous-pa764
(Solved): Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous ...
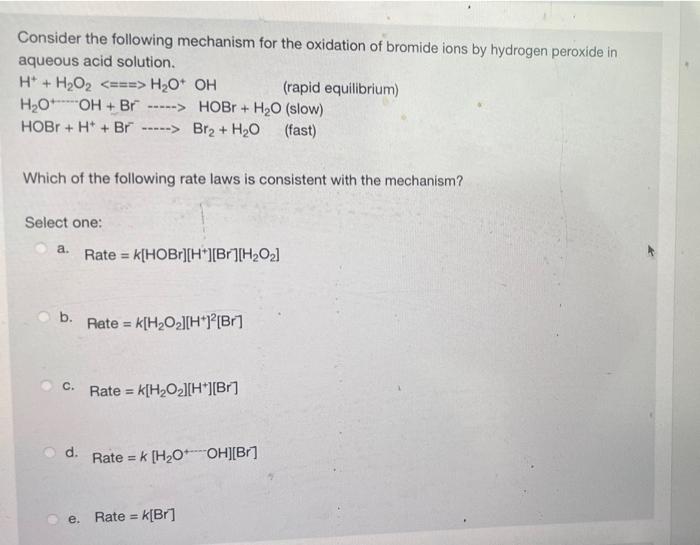
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. H + H?O? <===> H?O+ OH H?O OH+Br -----> HOBr + H?O (slow) HOBr + H+ + Br -----> Br? + H?O (fast) Which of the following rate laws is consistent with the mechanism? Select one: a. Rate = K[HOBr][H*][Br][H?0?] b. C. d. e. Rate = K[H?O?][H*]2[Br] Rate = k[H?O?][H*][Br] Rate = k [H?O OH][Br] (rapid equilibrium) Rate = k[Br]
Expert Answer
Remember when a certain reaction occours in multiple step , the step with the slowest r