Home /
Expert Answers /
Chemistry /
consider-the-equilibrium-system-described-by-the-chemical-reaction-below-for-this-reaction-m-pa754
(Solved): Consider the equilibrium system described by the chemical reaction below. For this reaction, \( \m ...
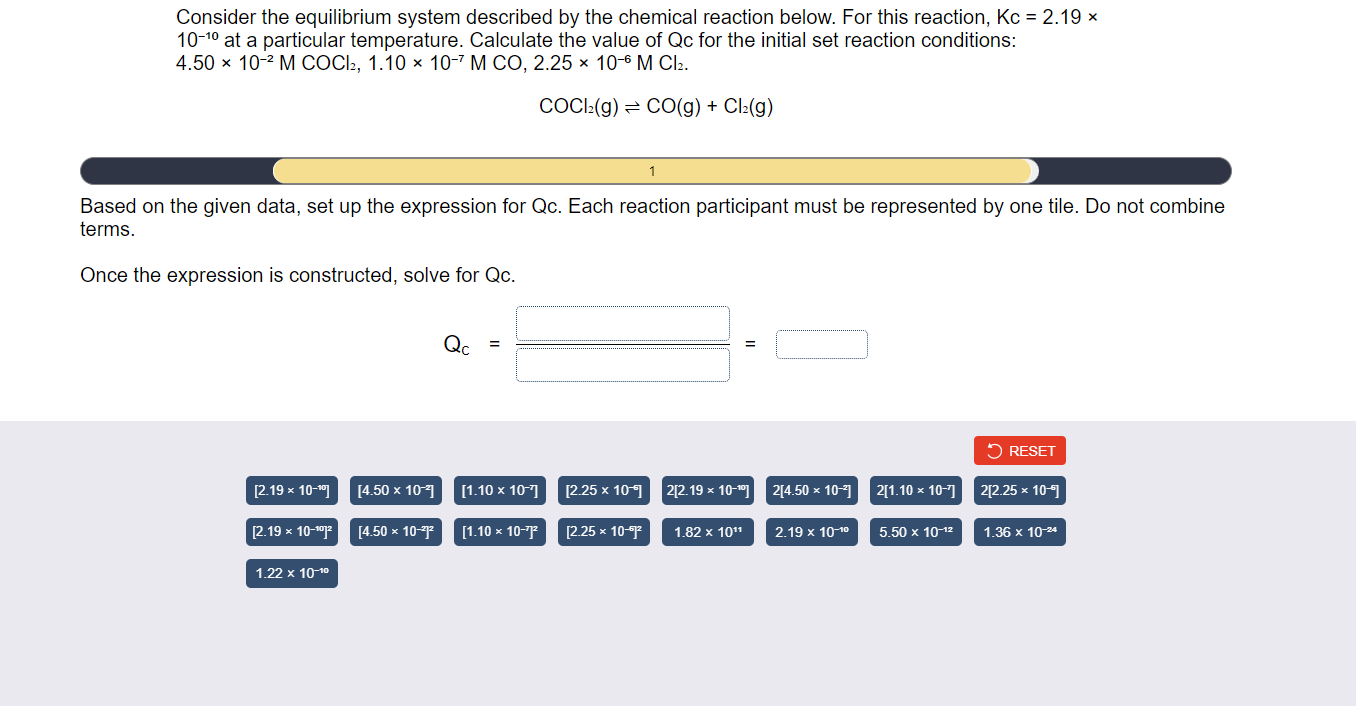
Consider the equilibrium system described by the chemical reaction below. For this reaction, \( \mathrm{Kc}=2.19 \times \) \( 10^{-10} \) at a particular temperature. Calculate the value of \( Q c \) for the initial set reaction conditions: \( 4.50 \times 10^{-2} \mathrm{M} \mathrm{COCl}_{2}, 1.10 \times 10^{-7} \mathrm{M} \mathrm{CO}, 2.25 \times 10^{-6} \mathrm{M} \mathrm{Cl}_{2} \). \[ \mathrm{COCl}_{2}(\mathrm{~g}) \rightleftharpoons \mathrm{CO}(\mathrm{g})+\mathrm{Cl}_{2}(\mathrm{~g}) \] Based on the given data, set up the expression for Qc. Each reaction participant must be represented by one tile. Do not combine erms. Once the expression is constructed, solve for Qc. \[ Q_{C}=\bar{\square}= \]
Expert Answer
The above problem is regarding Equilibrium constant (Keq) of a reaction and its relationship with Reaction quotient (Q) Both are give