Home /
Expert Answers /
Chemistry /
consider-the-energy-diagram-for-the-reaction-a-g-rightarrow-b-g-assume-the-relative-ener-pa268
(Solved): Consider the energy diagram for the reaction \( A(g) \rightarrow B(g) \). (Assume the relative ener ...
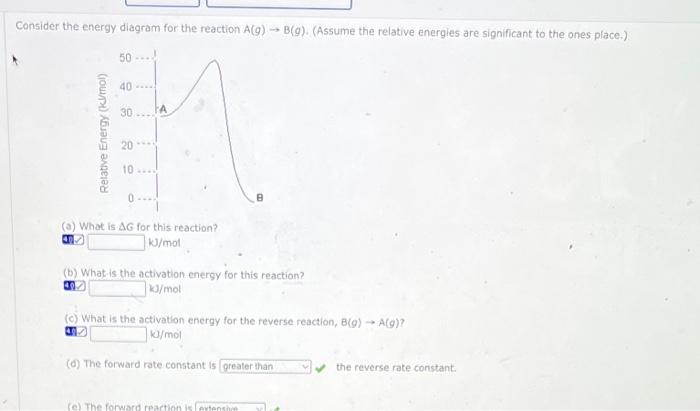
Consider the energy diagram for the reaction \( A(g) \rightarrow B(g) \). (Assume the relative energies are significant to the ones place.) (a) What is \( \Delta G \) for this reaction? ??? \( \mathrm{kJ} / \mathrm{mol} \) (b) What is the activation energy for this reaction? an \( \mathrm{kJ} / \mathrm{mol} \) (c) What is the activation energy for the reverse reaction, \( B(g) \rightarrow A(g) \) ? ?] \( \mathrm{kJ} / \mathrm{mol} \) (d) The forward rate constant is the reverse rate coristant.