Home /
Expert Answers /
Chemistry /
consider-the-decomposition-of-hydrogen-peroxide-h2o2-to-form-h2o-and-o-a-write-a-pa303
(Solved): Consider the decomposition of hydrogen peroxide (H2O2) to form H2O and O. (a) Write a ...
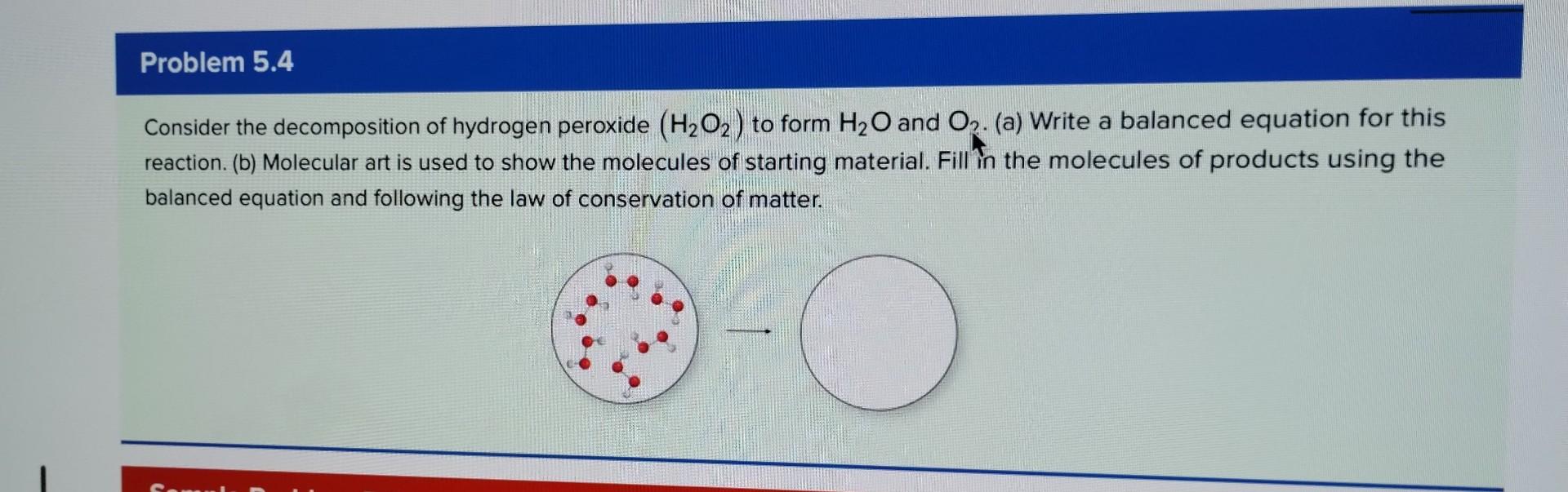
Consider the decomposition of hydrogen peroxide to form and . (a) Write a balanced equation for this reaction. (b) Molecular art is used to show the molecules of starting material. Fill in the molecules of products using the balanced equation and following the law of conservation of matter.
Expert Answer
Consider the decomposition of hydrogen peroxide(H2?O2?)to formH2?OandO. (a) Write a balanced equation for this reaction. (b) Molecular art is used to show the molecules of starting material. Fill in the molecules of products using the balanced equation and following the law of conservation of matter.