Home /
Expert Answers /
Chemistry /
consider-that-a-single-box-represents-an-orbital-and-an-electron-is-represented-as-a-half-arrow-o-pa612
(Solved): Consider that a single box represents an orbital, and an electron is represented as a half arrow. O ...
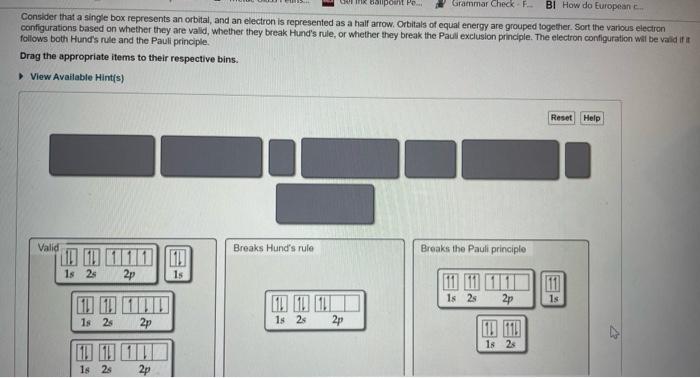
Consider that a single box represents an orbital, and an electron is represented as a half arrow. Orbitals of equat energy are grouped togetier. Sort the various electicn configurations based on whether they are valid, whether they break Hund's rule, or whether thay break the Paull exclusion principle. The electron configuration wil be valid if if follows both Hund's rule and the Pauli principle. Drag the appropriate items to their respective bins.