Home /
Expert Answers /
Chemistry /
consider-a-transition-of-the-electron-in-the-hydrogen-atom-from-n-3-to-n-7-is-del-pa977
(Solved): Consider a transition of the electron in the hydrogen atom from \( n=3 \) to \( n=7 \). Is \( \Del ...
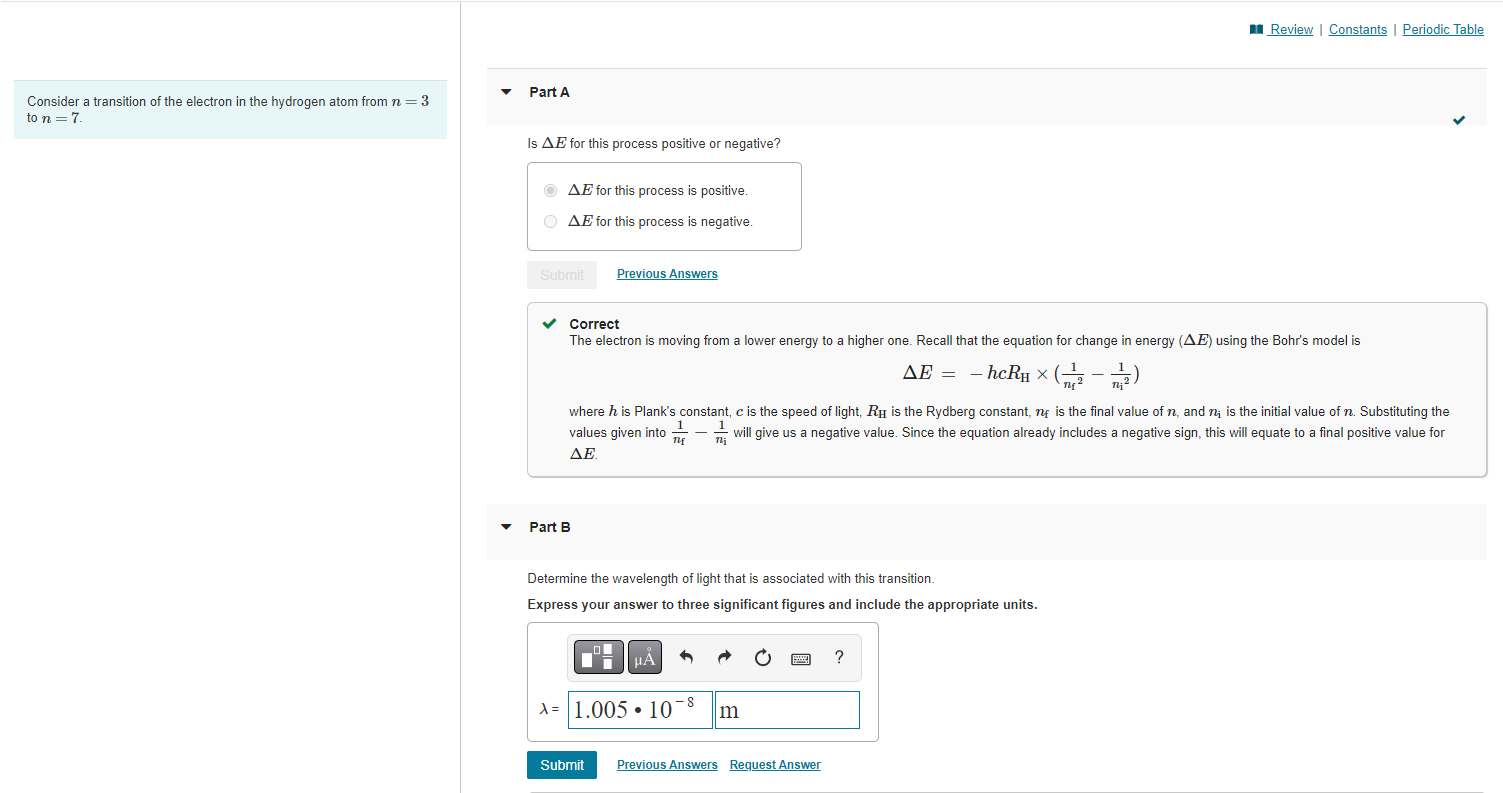
Consider a transition of the electron in the hydrogen atom from \( n=3 \) to \( n=7 \). Is \( \Delta E \) for this process positive or negative? \( \Delta E \) for this process is positive. \( \Delta E \) for this process is negative. Correct The electron is moving from a lower energy to a higher one. Recall that the equation for change in energy \( (\Delta E) \) using the Bohr's model is \[ \Delta E=-h c R_{\mathrm{H}} \times\left(\frac{1}{n_{\mathrm{f}}{ }^{2}}-\frac{1}{n_{\mathrm{i}}{ }^{2}}\right) \] where \( h \) is Plank's constant, \( c \) is the speed of light, \( R_{\mathrm{H}} \) is the Rydberg constant, \( n_{\mathrm{f}} \) is the final value of \( n \), and \( n_{\mathrm{i}} \) is the initial value of \( n \). Substituting the values given into \( \frac{1}{n_{\mathrm{f}}}-\frac{1}{n_{\mathrm{i}}} \) will give us a negative value. Since the equation already includes a negative sign, this will equate to a final positive value for Part B Determine the wavelength of light that is associated with this transition. Express your answer to three significant figures and include the appropriate units.
Expert Answer
We have equation, E = - 2.178 10 -18 J ( Z 2 / n 2 ) Where, Z is nuclear charge and n is integer. For Hydrogen atom, Z= 1. Energy corresponding to sta