Home /
Expert Answers /
Chemistry /
consider-a-galvanic-cell-with-two-half-cells-the-first-half-cell-consists-of-a-silver-electrode-pl-pa537
(Solved): Consider a galvanic cell with two half cells. The first half cell consists of a silver electrode pl ...
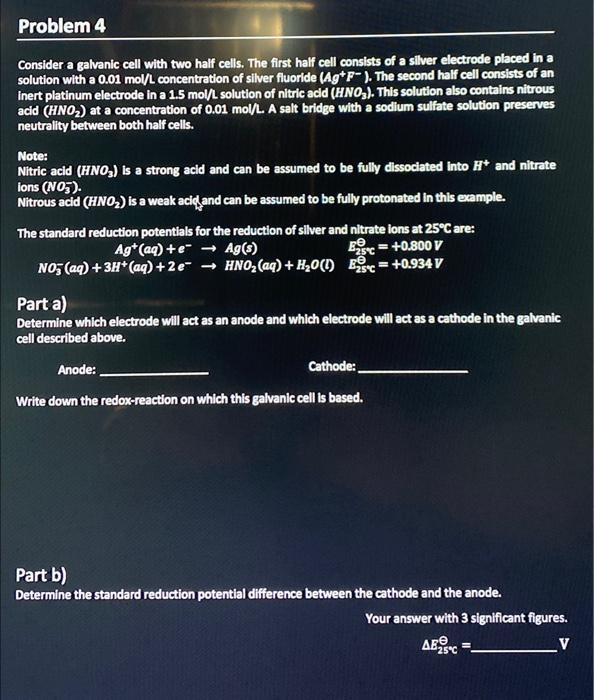
Consider a galvanic cell with two half cells. The first half cell consists of a silver electrode placed in a solution with a \( 0.01 \) mol/ concentration of silver fluoride \( \left(\mathrm{Ag}^{+} \mathrm{F}^{-}\right) \). The second half cell consists of an inert platinum electrode in a \( 1.5 \mathrm{~mol} / \mathrm{L} \) solution of nitric acid \( \left(\mathrm{HNO}_{3}\right. \). This solution also contains nitrous acid \( \left(\mathrm{HNO}_{2}\right) \) at a concentration of \( 0.01 \mathrm{~mol} / \mathrm{L} \). A salt bridge with a sodium sulfate solution preserves neutrality between both half cells. Noter Nitric acid \( \left(\mathrm{HNO}_{3}\right) \) is a strong acld and can be assumed to be fully dissociated into \( \mathrm{H}^{+} \)and nitrate ions \( \left(\mathrm{NO}_{3}\right) \). Nitrous acid \( \left(\mathrm{HNO}_{2}\right) \) is a weak acid and can be assumed to be fully protonated in this example. The standard reduction potentials for the reduction of silver and nitrate ions at \( 25^{\circ} \mathrm{C} \) are: \[ \begin{array}{cl} \mathrm{Ag}^{+}(a q)+e^{-} \rightarrow A g(s) & \mathrm{E}_{25 \%}=+0.800 \mathrm{~V} \\ \mathrm{NO}_{3}^{-}(a q)+3 \mathrm{H}^{+}(a q)+2 e^{-} \rightarrow \mathrm{HNO}_{2}(a q)+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) & \mathrm{E}_{25+\mathrm{C}}=+0.934 \mathrm{~V} \end{array} \] Part a) Determine which electrode will act as an anode and which electrode will act as a cathode in the galvanic cell described above. Anode: Cathode: Write down the redox-reaction on which this galvanic cell is based. Part b) Determine the standard reduction potential difference between the cathode and the anode. Your answer with 3 significant figures. \[ \Delta E_{2 S^{\circ} \mathrm{C}}^{\Theta}= \]
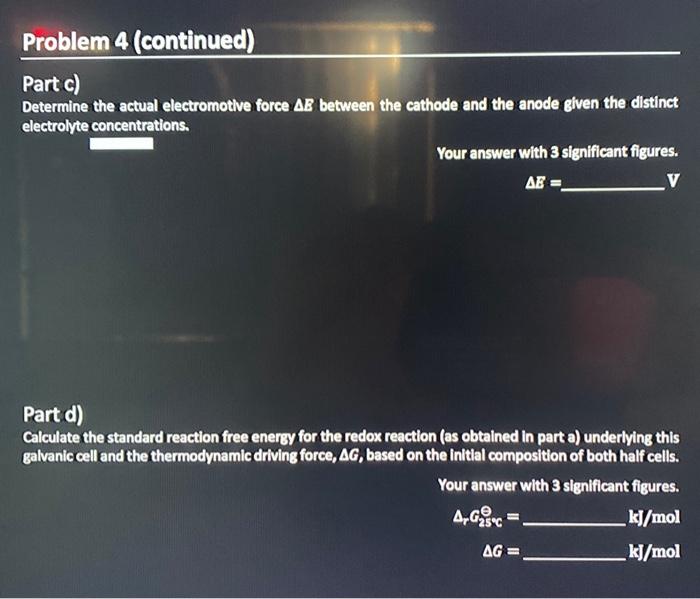
Part c) Determine the actual electromotive force \( \Delta B \) between the cathode and the anode given the distinct electrolyte concentrations. Your answer with 3 significant figures. \[ \Delta E= \] Part d) Calculate the standard reaction free energy for the redox reaction (as obtained in part a) underying this galvanic cell and the thermodynamic driving force, \( \Delta G \), based on the intital composition of both half cells. Your answer with 3 significant figures. \( \Delta_{r} c_{25 \times c}^{\theta}= \) \( \mathrm{kj} / \mathrm{mol} \) \( \Delta G= \) \( \mathrm{kj} / \mathrm{mol} \)