Home /
Expert Answers /
Chemistry /
complete-the-table-below-for-calculating-the-molar-mass-of-the-compound-silicon-dioxide-molar-mass-pa858
(Solved): Complete the table below for calculating the molar mass of the compound silicon dioxide. Molar mass ...
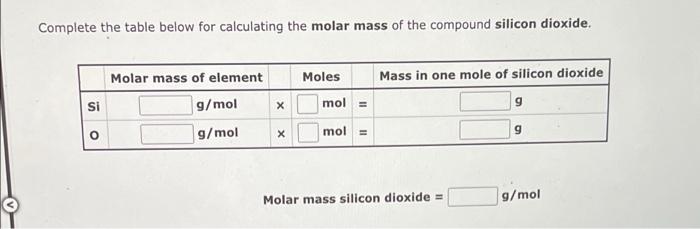


Complete the table below for calculating the molar mass of the compound silicon dioxide. Molar mass of element Moles Mass in one mole of silicon dioxide g/mol 9 g/mol Si mol = X mol = X Molar mass silicon dioxide = 9 g/mol
A 23.60 gram sample of iron is heated in the presence of excess fluorine. A metal fluoride is formed with a mass of 47.69 g. Determine the empirical formula of the metal fluoride. Enter the elements in the order Fe, F empirical formula =
A 12.04 gram sample of copper is heated in the presence of excess fluorine. A metal fluoride is formed with a mass of 15.64 g. Determine the empirical formula of the metal fluoride. Enter the elements in the order Cu, F empirical formula =