Home /
Expert Answers /
Chemistry /
combustion-of-hydrocarbons-such-as-propane-c3h8-produces-carbon-dioxide-a-34-greenhouse-gas-pa273
(Solved): Combustion of hydrocarbons such as propane (C3H8) produces carbon dioxide, a "greenhouse gas. ...
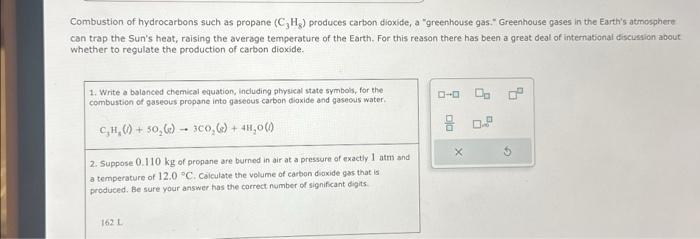
Combustion of hydrocarbons such as propane produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discishion about whether to regulate the production of carbon dioxide. 1. Write a bolanced chemical equation, including physical state symbois, for the combustion of gaseous propane into gaseous carbon dioxide and gaseous water. 2. Suppose of propane are burned in air at a pressure of exactly and a temperature of . Caiculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits