Home /
Expert Answers /
Chemistry /
classify-each-titration-curve-as-representing-a-strong-acid-titrated-with-a-strong-base-a-strong-pa983
(Solved): Classify each titration curve as representing a strong acid titrated with a strong base, a strong ...
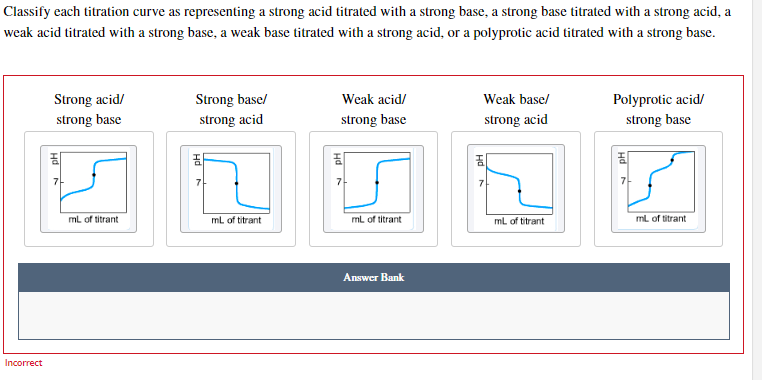
Classify each titration curve as representing a strong acid titrated with a strong base, a strong base titrated with a strong acid, a veak acid titrated with a strong base, a weak base titrated with a strong acid, or a polyprotic acid titrated with a strong base.
Expert Answer
The initial pH value of the solution shows that the weak acid is titrated against the strong base because the pH value after the complete reaction is very high. So, the first titration curve is