Home /
Expert Answers /
Chemistry /
classify-each-reactant-in-the-chemical-equations-as-an-oxidizing-agent-a-reducing-agent-or-neithe-pa745
(Solved): Classify each reactant in the chemical equations as an oxidizing agent, a reducing agent, or neithe ...
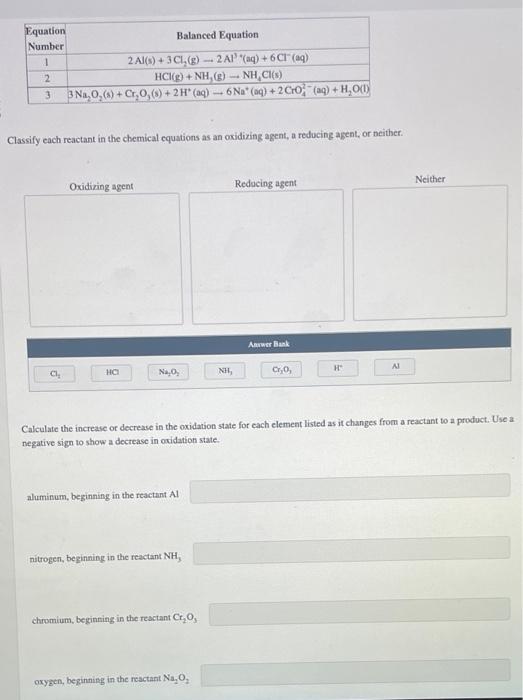
Classify each reactant in the chemical equations as an oxidizing agent, a reducing agent, or neither. Calculate the increase or decrease in the oxidation state for each element listed as it changes from a reactant to a product. Use a negative sign to show a decrease in oxidation state. aluminum, beginning in the reactant \( \mathrm{Al} \) nitrogen, beginning in the reactant \( \mathrm{NH}_{3} \). chromium, beginning in the reactant \( \mathrm{Cr}_{2} \mathrm{O}_{3} \). oxyeen, beginning in the reactant \( \mathrm{Na}_{2} \mathrm{O}_{2} \)