Home /
Expert Answers /
Chemistry /
chlorine-gas-can-be-prepared-in-the-laboratory-by-the-reaction-of-hydrochloric-acid-with-manganese-pa734
(Solved): Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese ...
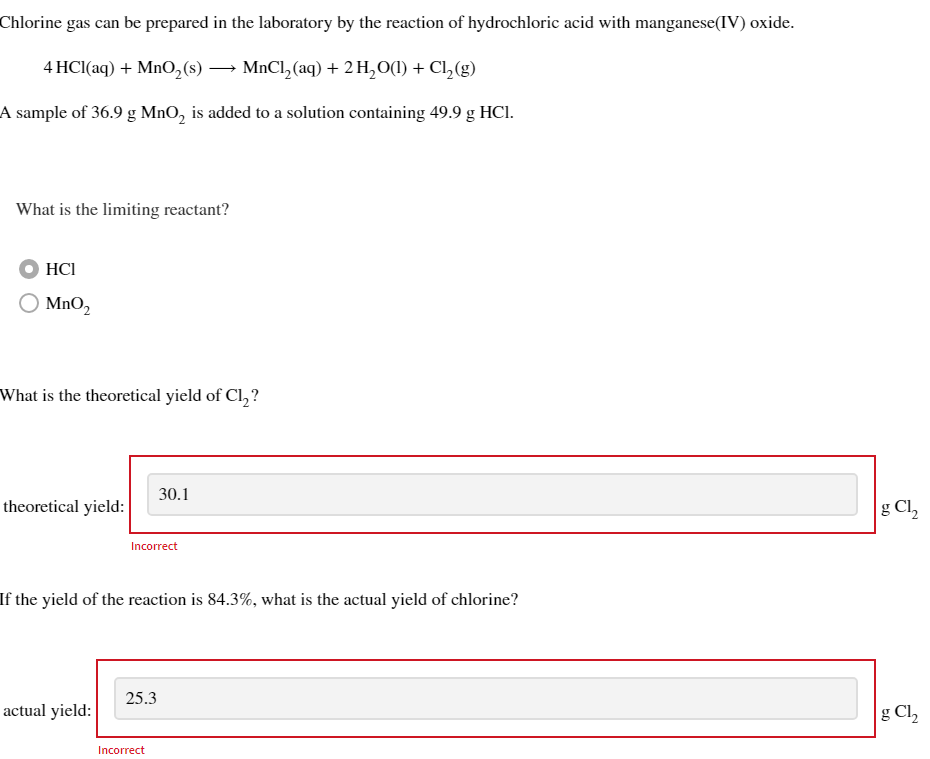
Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese(IV) oxide. \[ 4 \mathrm{HCl}(\mathrm{aq})+\mathrm{MnO}_{2}(\mathrm{~s}) \longrightarrow \mathrm{MnCl}_{2}(\mathrm{aq})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})+\mathrm{Cl}_{2}(\mathrm{~g}) \] A sample of \( 36.9 \mathrm{~g} \mathrm{MnO}_{2} \) is added to a solution containing \( 49.9 \mathrm{~g} \mathrm{HCl} \). What is the limiting reactant? \[ \begin{array}{l} \mathrm{HCl} \\ \mathrm{MnO}_{2} \end{array} \] What is the theoretical yield of \( \mathrm{Cl}_{2} \) ? theoretical yie Incorrect If the yield of the reaction is \( 84.3 \% \), what is the actual yield of chlorine?