Home /
Expert Answers /
Chemistry /
chem-2-help-please-nbsp-how-to-use-graphical-analysis-to-draw-graphs-8-points-give-the-chemical-f-pa831
(Solved): Chem 2 help please How to Use Graphical Analysis to Draw Graphs 8 points) Give the chemical f ...
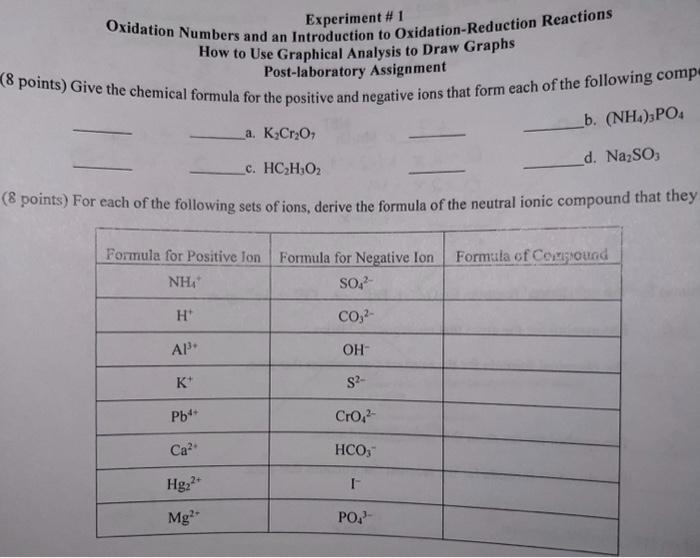
![\[
\begin{array}{l}
0=2 x+(-2 \times 7) \\
0=2 x-14 \\
+\frac{14}{0}=2 x=+7=x
\end{array}
\]
6. (12 points) For the unbalance](https://media.cheggcdn.com/study/e86/e8614997-c01f-4c25-99f5-bb37635b9732/image)
Chem 2 help please
How to Use Graphical Analysis to Draw Graphs 8 points) Give the chemical formula for the positive and negative ions that form each of the following comp a. \( \mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O} \), b. \( \left(\mathrm{NH}_{4}\right)_{3} \mathrm{PO}_{4} \) c. \( \mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2} \) d. \( \mathrm{Na}_{2} \mathrm{SO}_{3} \) 8 points) For each of the following sets of ions, derive the formula of the neutral ionic compound that they
\[ \begin{array}{l} 0=2 x+(-2 \times 7) \\ 0=2 x-14 \\ +\frac{14}{0}=2 x=+7=x \end{array} \] 6. (12 points) For the unbalanced reaction, \( \mathrm{Cr}_{2} \mathrm{O}_{2}^{2}+\mathrm{Fe}^{2+} \rightarrow \mathrm{Cr}^{30}+\mathrm{Fe}^{3} \), give: \( \mathrm{Fe} \) The symbol for the atom that undergoes oxidation. The chemical formula for the reactant that contains that atom. C. The symbol for the atom that undergoes reduction. The chemical formula for the reactant that contains that atom. Based on your previous answers: is the oxidizing agent. is the reducing agent. 7. ( 6 points) Mark with an \( \mathrm{X} \) the reaction(s) that is/are oxidation-reduction reactions. \[ \mathrm{NO}_{2}^{-}+\mathrm{MnO}_{4}^{-} \rightarrow \mathrm{NO}_{3}^{-}+\mathrm{MnO}_{2} \] \( \mathrm{Zn}+2 \mathrm{HCl} \rightarrow \mathrm{H}_{2}+\mathrm{ZnCl}_{2} \) \( \mathrm{CdCl}_{2}+2 \mathrm{NaOH} \rightarrow \mathrm{Cd}(\mathrm{OH})_{2}+2 \mathrm{NaCl} \)
Expert Answer
The cation (positive ion) and anion (negative ion) in the following compounds are K+ (cation) and Cr2O72- (anion) in K2Cr2O7 NH4+ (cation) and PO43- (