Home /
Expert Answers /
Chemistry /
can-you-write-a-chemical-equation-where-you-turn-benzil-into-hydrobenzoin-over-the-arrow-include-na-pa933
(Solved): can you write a chemical equation where you turn benzil into hydrobenzoin. over the arrow include Na ...
can you write a chemical equation where you turn benzil into hydrobenzoin. over the arrow include NaBH4 and heat and water
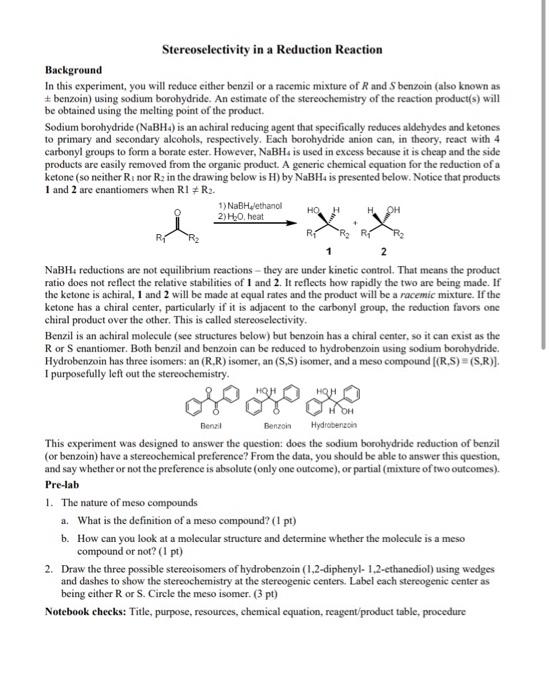

Background In this experiment, you will reduce either benzil or a racemic mixture of \( R \) and \( S \) benzoin (also known as \( \pm \) benzoin) using sodium borohydride. An estimate of the stereochemistry of the reaction product(s) will be obtained using the melting point of the product. Sodium borohydride \( \left(\mathrm{NaBH}_{4}\right) \) is an achiral reducing agent that specifically reduces aldehydes and ketones to primary and secondary alcohols, respectively. Each borohydride anion can, in theory, react with 4 carbonyl groups to form a borate ester. However, \( \mathrm{NaBH}_{4} \) is used in excess because it is cheap and the side products are easily removed from the organic product. A generic chemical equation for the reduction of a ketone (so neither \( \mathrm{R}_{1} \) nor \( \mathrm{R}_{2} \) in the drawing below is \( \mathrm{H} \) ) by \( \mathrm{NaBH}_{4} \) is presented below. Notice that products 1 and 2 are enantiomers when \( \mathrm{R} 1 \neq \mathrm{R}_{2} \). \( \mathrm{NaBH}_{4} \) reductions are not equilibrium reactions - they are under kinetic control. That means the product ratio does not reflect the relative stabilities of 1 and 2 . It reflects how rapidly the two are being made. If the ketone is achiral, 1 and 2 will be made at equal rates and the product will be a racemic mixture. If the ketone has a chiral center, particularly if it is adjacent to the carbonyl group, the reduction favors one chiral product over the other. This is ealled stereoselectivity. Benzil is an achiral molecule (see structures below) but benzoin has a chiral center, so it can exist as the \( \mathrm{R} \) or \( \mathrm{S} \) enantiomer. Both benzil and benzoin can be reduced to hydrobenzoin using sodium borohydride. Hydrobenzoin has three isomers: an \( (R, R) \) isomer, an \( (S, S) \) isomer, and a meso compound \( [(R, S)=(S, R)] \). I purposefully left out the stereochemistry. This experiment was designed to answer the question: does the sodium borohydride reduction of benzil (or benzoin) have a stereochemical preference? From the data, you should be able to answer this question, and say whether or not the preference is absolute (only one outcome), or partial (mixture of two outcomes). Pre-lab 1. The nature of meso compounds a. What is the definition of a meso compound? (1 pt) b. How can you look at a molecular structure and determine whether the molecule is a meso compound or not? (1 pt) 2. Draw the three possible stereoisomers of hydrobenzoin (1,2-diphenyl-1,2-ethanediol) using wedges and dashes to show the stereochemistry at the stereogenic centers. Label each stercogenic center as being either \( \mathrm{R} \) or \( \mathrm{S} \). Circle the meso isomer. (3 pt) Notebook checks: Title, purpose, resources, chemical equation, reagent/product table, procedure