Home /
Expert Answers /
Chemistry /
can-you-please-show-work-thank-you-will-give-thumbs-up-1-dinitrogen-monoxide-aka-nitrous-oxide-i-pa993
(Solved): can you please show work, thank you will give thumbs up 1. Dinitrogen monoxide, aka nitrous oxide, i ...

can you please show work, thank you will give thumbs up
1. Dinitrogen monoxide, aka nitrous oxide, is manufactured by heating ammonium nitrate until it decomposes in the following unbalanced chemical equation. \[ \mathrm{NH}_{4} \mathrm{NO}_{3}(\mathrm{~s}) \rightarrow \mathrm{N}_{2} \mathrm{O}(\mathrm{g})+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \] A. Balance the equation. B. Calculate the mass of ammonium nitrate needed to produce \( 10.0 \mathrm{~g} \) of nitrous oxide. C. Determine the mass of water produced in the reaction described in Part \( \mathrm{B} \). D. If \( 23.5 \mathrm{~g} \) of ammonium nitrate decomposes, how much nitrous oxide would be produced?
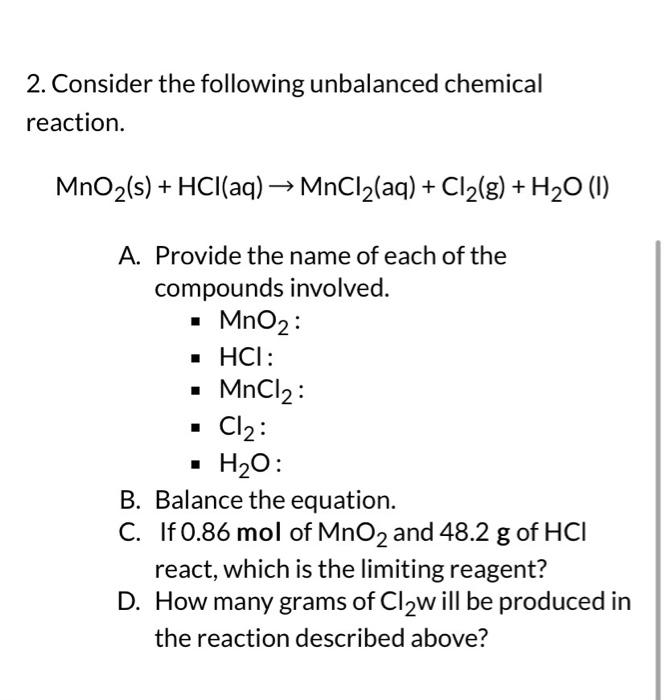
2. Consider the following unbalanced chemical reaction. \[ \mathrm{MnO}_{2}(\mathrm{~s})+\mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{MnCl}_{2}(\mathrm{aq})+\mathrm{Cl}_{2}(\mathrm{~g})+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \] A. Provide the name of each of the compounds involved. - \( \mathrm{MnO}_{2} \) : - \( \mathrm{HCl} \) : - \( \mathrm{MnCl}_{2} \) : - \( \mathrm{Cl}_{2} \) : - \( \mathrm{H}_{2} \mathrm{O} \) : B. Balance the equation. C. If \( 0.86 \mathrm{~mol} \) of \( \mathrm{MnO}_{2} \) and \( 48.2 \mathrm{~g} \) of \( \mathrm{HCl} \) react, which is the limiting reagent? D. How many grams of \( \mathrm{Cl}_{2} \mathrm{w} \) ill be produced in the reaction described above?