Home /
Expert Answers /
Chemistry /
calculate-the-standard-entropy-of-formation-in-mathrm-j-mathrm-mol-pa347
(Solved): Calculate the standard entropy of formation (in \( \mathrm{J} \mathrm{mol}^{- ...
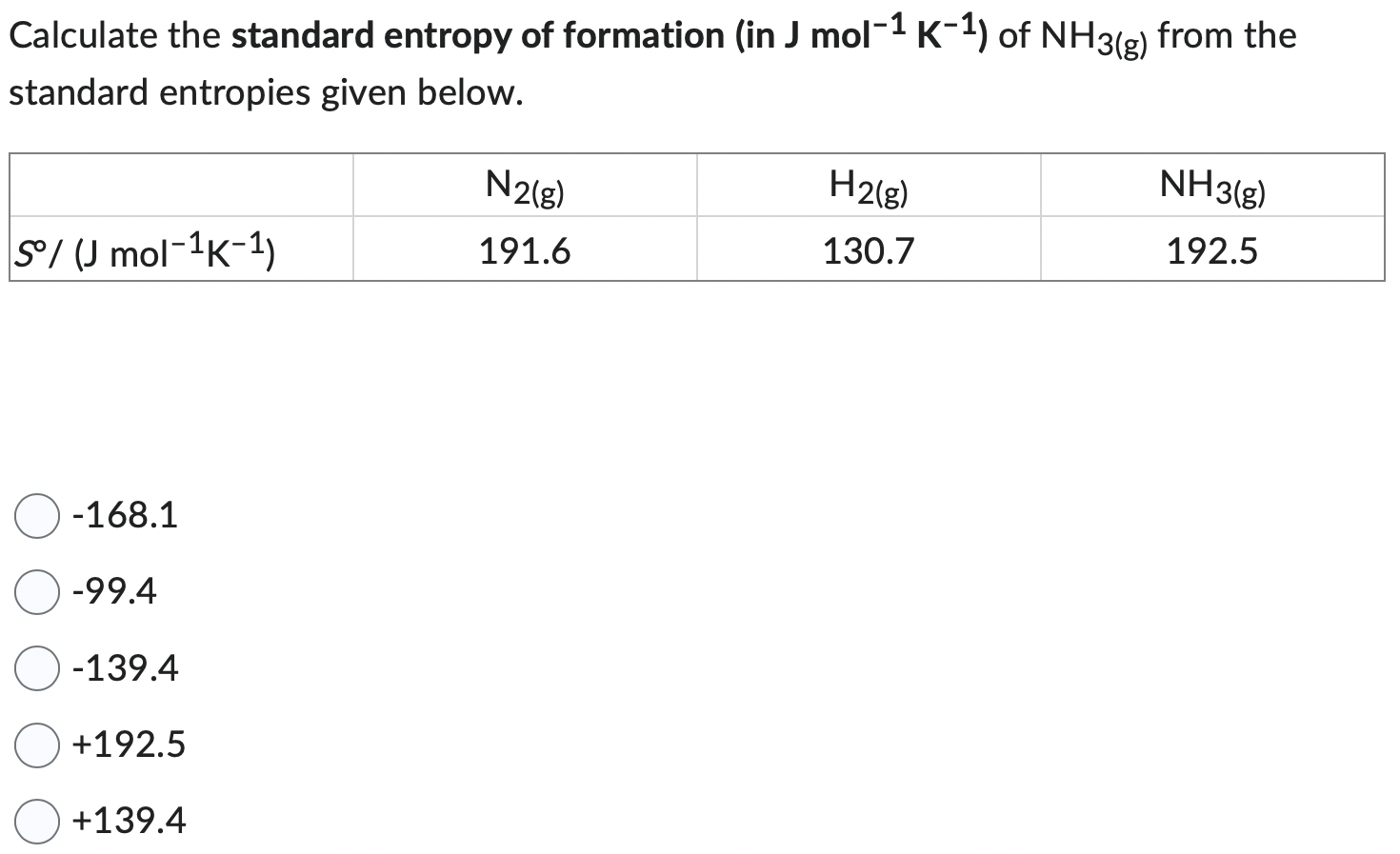
Calculate the standard entropy of formation (in \( \mathrm{J} \mathrm{mol}^{-1} \mathrm{~K}^{-1} \) ) of \( \mathrm{NH}_{3(\mathrm{~g})} \) from the standard entropies given below. \[ \begin{array}{c} -168.1 \\ -99.4 \\ -139.4 \\ +192.5 \\ +139.4 \end{array} \]