Home /
Expert Answers /
Chemistry /
calculate-the-mass-of-clove-oil-present-in-4-g-of-cloves-and-the-theoretical-mass-of-clove-oil-you-c-pa695
(Solved): Calculate the mass of clove oil present in 4 g of cloves and the theoretical mass of clove oil you c ...
Calculate the mass of clove oil present in 4 g of cloves and the
theoretical mass of clove oil you
could isolate from the cloves by steam distillation. And the
theoretical yield of clove oil.
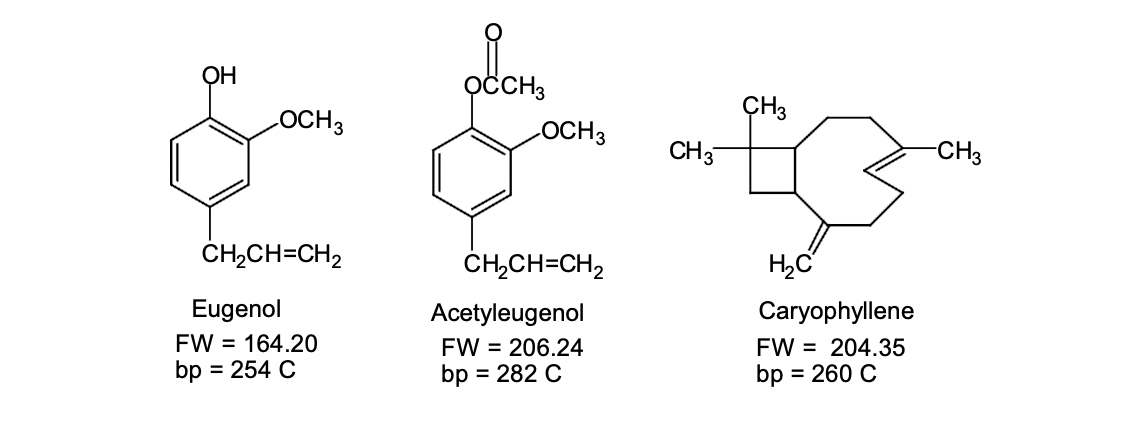
Eugenol \( \mathrm{FW}=164.20 \) \( \mathrm{bp}=254 \mathrm{C} \) Acetyleugenol \( F W=206.24 \) \( b p=282 \mathrm{C} \) Caryophyllene \( \mathrm{FW}=204.35 \) \( \mathrm{bp}=260 \mathrm{C} \)
Expert Answer
Answer:- step by step Explanation Isolation of Eugenol from Cloves Objective: Clove oil is isolated from Cloves using the Steam Distillation technique. The clove oil's composition is analyzed. The percent recovery of clove oil is calculated. Gas Chro