Home /
Expert Answers /
Chemistry /
calculate-the-heat-of-reaction-delta-h-for-the-following-reaction-2-mathrm-nh-3-g-pa368
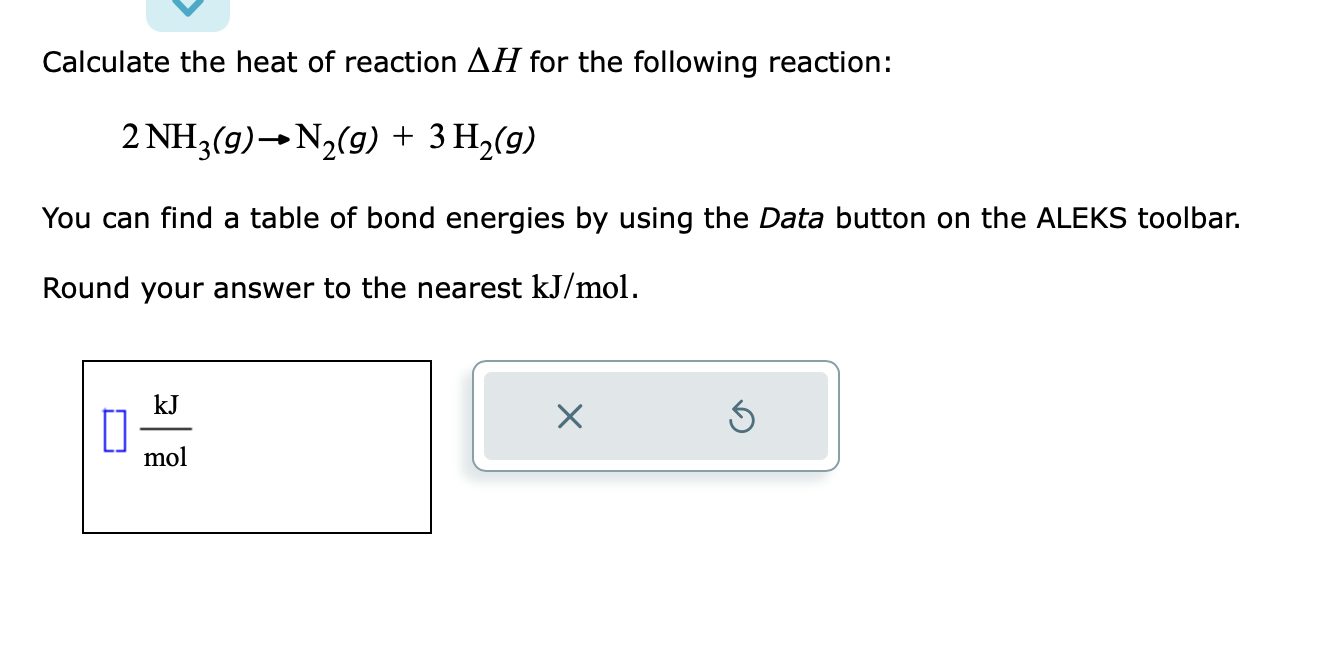
(Solved): Calculate the heat of reaction \( \Delta H \) for the following reaction: \[ 2 \mathrm{NH}_{3}(g) ...
Calculate the heat of reaction \( \Delta H \) for the following reaction: \[ 2 \mathrm{NH}_{3}(g) \rightarrow \mathrm{N}_{2}(g)+3 \mathrm{H}_{2}(g) \] You can find a table of bond energies by using the Data button on the ALEKS toolbar. Round your answer to the nearest \( \mathrm{kJ} / \mathrm{mol} \).
Expert Answer
Delta h formation for a reaction is given by = ( sum of m*enthalpy of formation of products - sum of n* enthalpy of