Home /
Expert Answers /
Chemistry /
calculate-the-enthalpy-of-the-reaction-below-ahrxn-in-kj-using-the-bond-energies-provided-co-g-pa992
(Solved): Calculate the enthalpy of the reaction below (AHrxn, in kJ) using the bond energies provided. CO(g) ...
Calculate the enthalpy of the reaction below (AHrxn, in kJ) using the bond energies provided. CO(g) + Cl₂(g) → CI₂CO(g). +CI-CI Bond Type CEO C=O C-CI CI-CI C-C Bond Energy (kJ/mol) 1072 799 339 243 de CI CI 346 1 4 7 +/- 2 5 8 kJ 3 6 (O 9 0
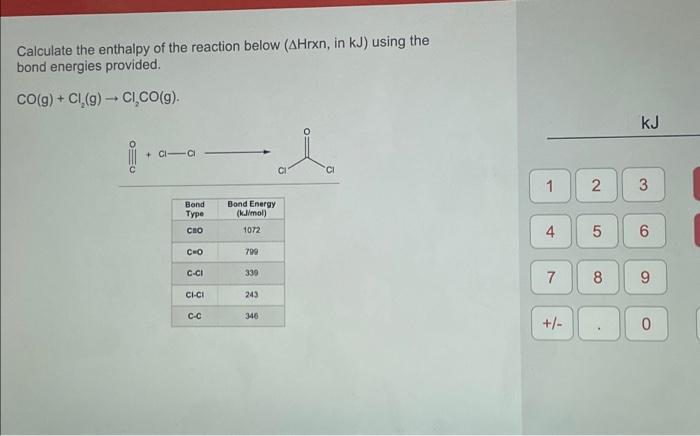
Calculate the enthalpy of the reaction below ( , in using the bond energies provided.