Home /
Expert Answers /
Chemistry /
calculate-the-change-in-standard-free-energy-for-electron-transfer-from-cytochorome-c-to-molecular-pa352
(Solved): Calculate the change in standard free energy for electron transfer from cytochorome c to molecular ...
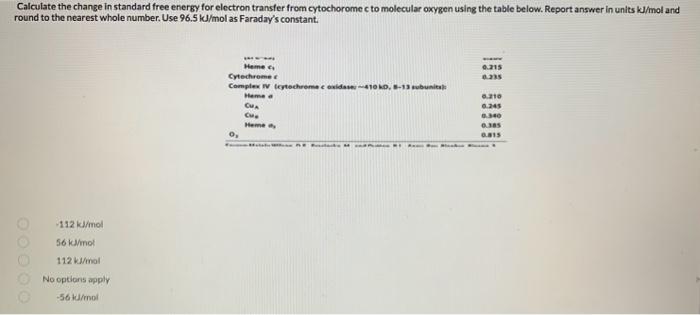

Calculate the change in standard free energy for electron transfer from cytochorome c to molecular oxygen using the table below. Report answer in units ku/mol and round to the nearest whole number. Use \( 96.5 \mathrm{~kJ} / \mathrm{mol} \) as Faraday's constant. \( -112 \mathrm{~kJ} / \mathrm{mol} \) \( 56 \mathrm{kJin} \) \( 112 \mathrm{kd} / \mathrm{mal} \) No options apply \( -561 \mathrm{id} / \mathrm{mol} \)
Heme \( c_{1} \) Cytochrome c Complex IV (cytochrome coxidases \( \sim 410 \mathrm{kD}, 8-13 \) subunits): Heme a \( \mathrm{Cu}_{\mathrm{A}} \) \( \mathrm{Cu}_{\mathrm{a}} \) Heme \( a_{3} \)