Home /
Expert Answers /
Chemistry /
calcium-sulfide-mathrm-cas-adopts-the-crystal-structure-shown-below-the-yellow-spheres-a-pa490
(Solved): Calcium sulfide \( (\mathrm{CaS}) \) adopts the crystal structure shown below. The yellow spheres a ...
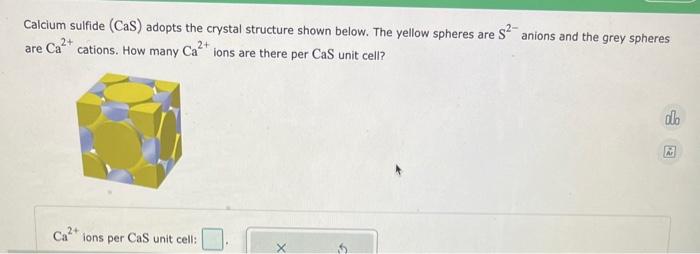
Calcium sulfide \( (\mathrm{CaS}) \) adopts the crystal structure shown below. The yellow spheres are \( \mathrm{S}^{2-} \) anions and the grey spheres are \( \mathrm{Ca}^{2+} \) cations. How many \( \mathrm{Ca}^{2+} \) ions are there per \( \mathrm{CaS} \) unit cell? \( \mathrm{Ca}^{2+} \) ions per \( \mathrm{CaS} \) unit cell: