Home /
Expert Answers /
Chemistry /
buffer-worksheet-nbsp-please-answer-the-missi-no-parts-of-the-table-thank-you-nbsp-1-data-3-conc-pa622
(Solved): buffer worksheet please answer the missi no parts of the table thank you 1. DATA 3. CONC ...
buffer worksheet
please answer the missi no parts of the table thank you


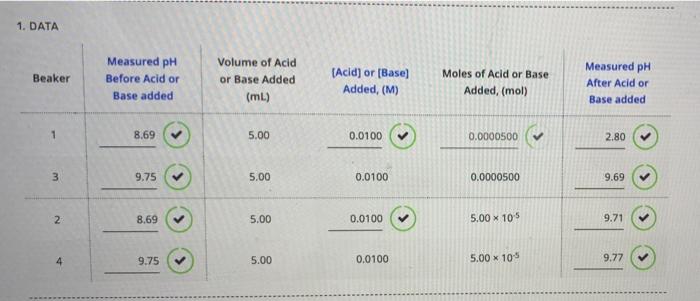
1. DATA
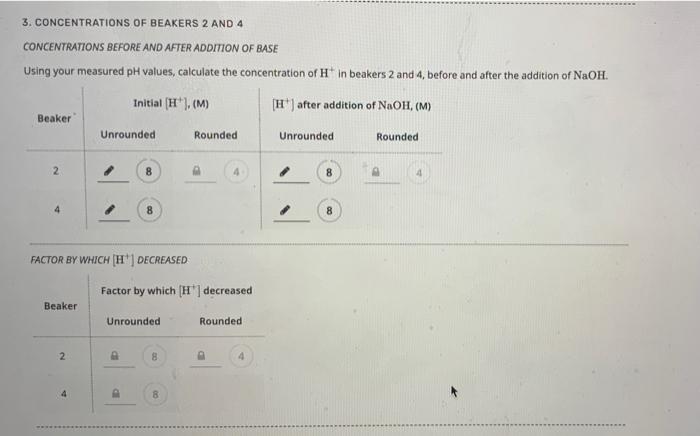
3. CONCENTRATIONS OF BEAKERS 2 AND 4 CONCENTRATIONS BEFORE AND AFTER ADDITION OF BASE Using your measured pH values, calculate the concentration of \( \mathrm{H}^{+} \)in beakers 2 and 4 , before and after the addition of \( \mathrm{NaOH} \).
Expert Answer
When pH is given we can calculate the concentration of H+ ion in following way, Concentration of H+ = 10-pH For Beaker #2 : pH before the addition of NaOH was added = 8.69 [H+] Concentration = 10-pH = 10-8.69 = 2.04174 x 10-9 = 2.04 x 10-9 ( rounded