Home /
Expert Answers /
Chemistry /
benzoic-acid-an-organic-acid-is-soluble-in-which-of-the-following-organic-solvents-diethyl-ether-pa477
(Solved): Benzoic acid (an organic acid) is soluble in which of the following organic solvents? Diethyl ether ...
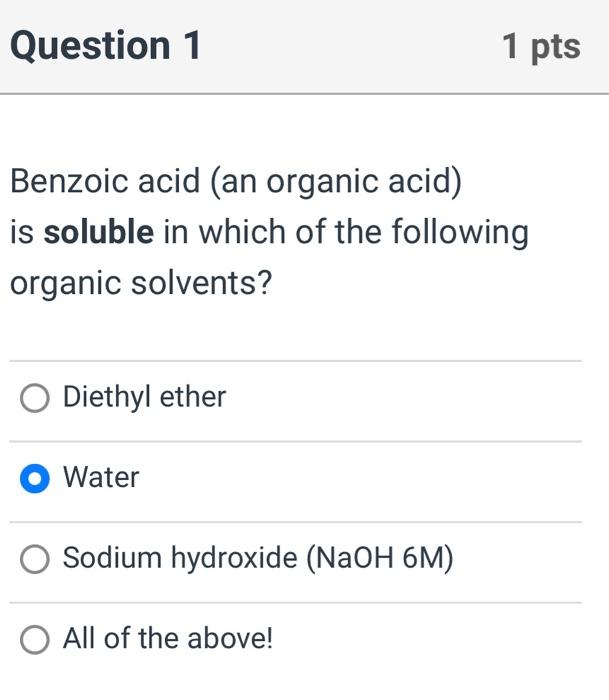

Benzoic acid (an organic acid) is soluble in which of the following organic solvents? Diethyl ether Water Sodium hydroxide ( \( \mathrm{NaOH} \) 6M) All of the above!
Suppose, the weight of the unknown mixture you started with is \( 142.3 \mathrm{mg} \). The weight of the extracted benzoic acid is \( 41.7 \mathrm{mg} \). The weight of the extracted naphthalene is \( 48.8 \mathrm{mg} \). You can combine the weights of the chemicals you isolated and compare that weight to the weight you started with to determine a percent recovery. Calculate the extraction recovery efficiency (i.e. percent recovery). Equation: \( \% \) recovery \( = \) (combined weight of the isolated compounds/weight of the starting mixture) \( \times 100 \) \begin{tabular}{l} \( 93.5 \% \) \\ \( 83.5 \% \) \\ \( 73.5 \% \) \\ \hline \( 63.5 \% \) \end{tabular}
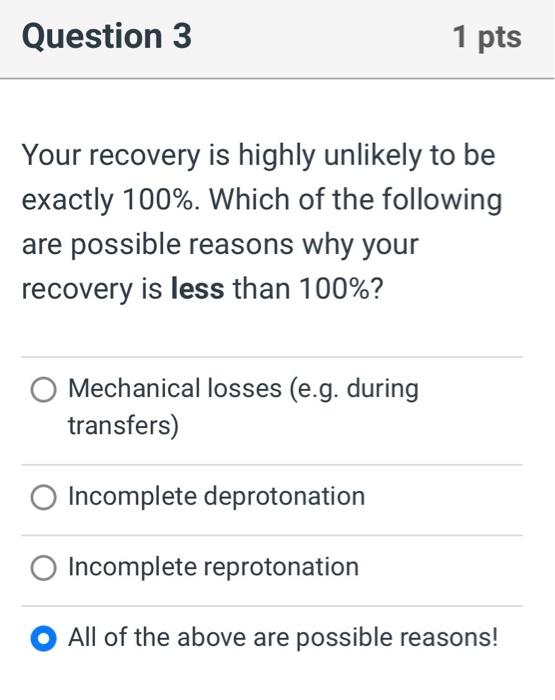
Your recovery is highly unlikely to be exactly \( 100 \% \). Which of the following are possible reasons why your recovery is less than \( 100 \% \) ? Mechanical losses (e.g. during transfers) Incomplete deprotonation Incomplete reprotonation All of the above are possible reasons!