Home /
Expert Answers /
Chemistry /
below-is-the-titration-curve-for-histidine-the-pka-values-are-1-8-cooh-6-0-side-chain-pa812
(Solved): Below is the titration curve for histidine. The pKa values are 1.8 (COOH), 6.0 (side chain) ...
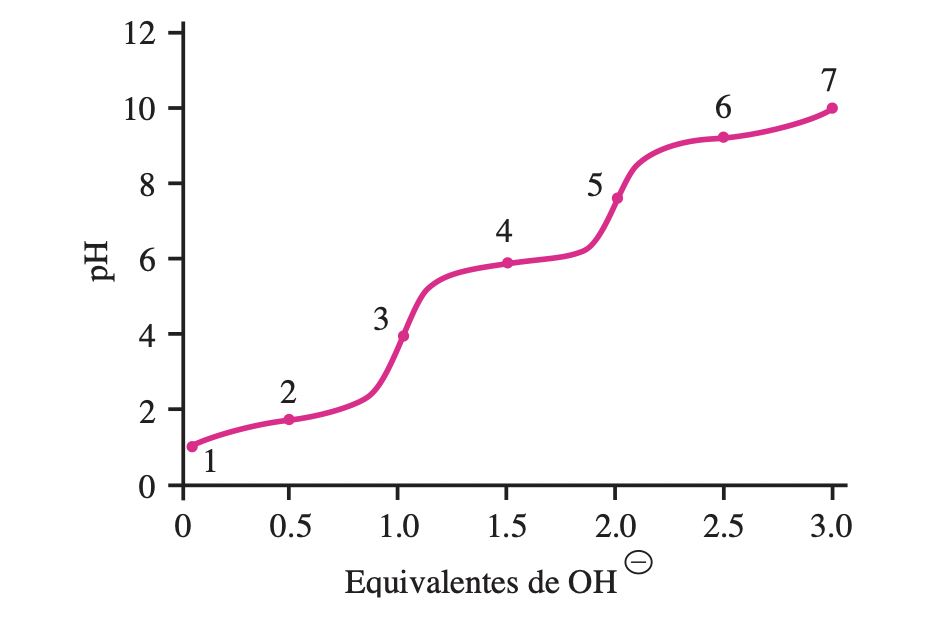
Below is the titration curve for histidine. The pKa values ??are 1.8 (—COOH), 6.0 (side chain), and 9.3 (—NH3?).
a) Draw the structure of histidine at each ionization
step.
b) On the titration curve, identify the points that correspond to
the four ionic species.
c) Identify the points where the average net charge is ˜2, ˜0.5, 0,
and ˜1.
d) Identify the point at which the pH equals the pKa of the side
chain.
e) Identify the point that indicates the complete titration of the
side chain.
f) Between what pH limits would histidine be a good buffer?