Home /
Expert Answers /
Chemistry /
below-is-a-solubility-curve-for-an-unknown-compound-a-suppose-15-grams-of-the-unknown-is-stirred-pa984
(Solved): Below is a solubility curve for an unknown compound. a. Suppose 15 grams of the unknown is stirred ...
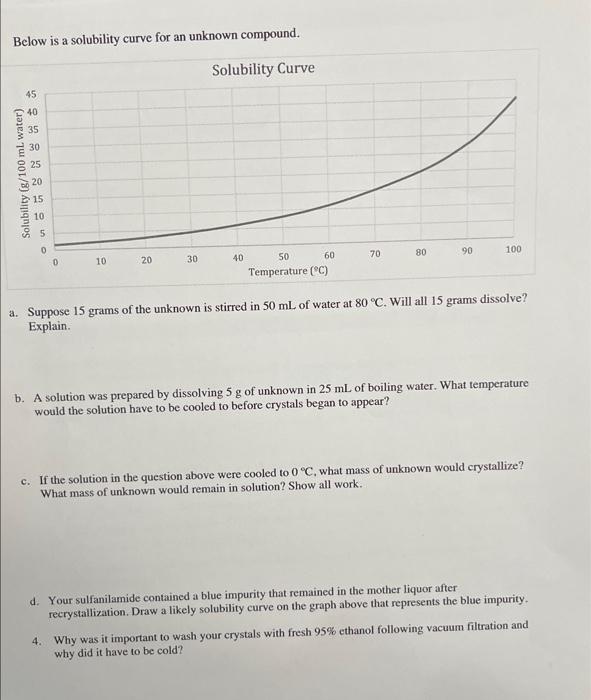
Below is a solubility curve for an unknown compound. a. Suppose 15 grams of the unknown is stirred in of water at . Will all 15 grams dissolve? Explain. b. A solution was prepared by dissolving of unknown in of boiling water. What temperature would the solution have to be cooled to before crystals began to appear? c. If the solution in the question above were cooled to , what mass of unknown would erystallize? What mass of unknown would remain in solution? Show all work. d. Your sulfanilamide contained a blue impurity that remained in the mother liquor after recrystallization. Draw a likely solubility curve on the graph above that represents the blue impurity. 4. Why was it important to wash your crystals with fresh ethanol following vacuum filtration and why did it have to be cold?
Expert Answer
A. No, not all 15 grams dissolve at 80°C. According to the solubility curve, the maximum solubility of the unknown is about 12 g/100 ml at 80°C. Since