Home /
Expert Answers /
Chemistry /
below-is-a-graph-of-mathrm-ph-vs-volume-of-mathrm-naoh-mathrm-ml-added-during-a-pa687
(Solved): Below is a graph of \( \mathrm{pH} \) vs. volume of \( \mathrm{NaOH}(\mathrm{mL}) \) added during a ...
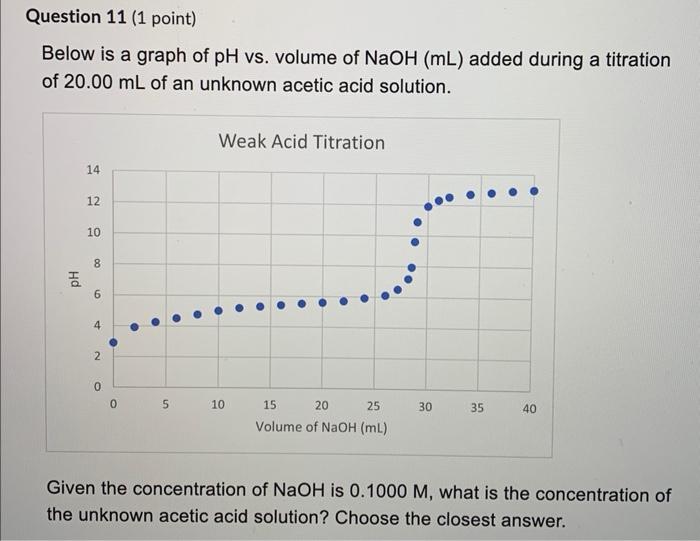

Below is a graph of \( \mathrm{pH} \) vs. volume of \( \mathrm{NaOH}(\mathrm{mL}) \) added during a titration of \( 20.00 \mathrm{~mL} \) of an unknown acetic acid solution. Given the concentration of \( \mathrm{NaOH} \) is \( 0.1000 \mathrm{M} \), what is the concentration of the unknown acetic acid solution? Choose the closest answer.
Given the concentration of \( \mathrm{NaOH} \) is \( 0.1000 \mathrm{M} \), what is the concentration of the unknown acetic acid solution? Choose the closest answer. \( 0.7000 \mathrm{M} \) \( 0.1400 \mathrm{M} \) \( 2.800 \mathrm{M} \) \( 0.2800 \mathrm{M} \) \( 0.07000 \mathrm{M} \) \( 1.000 \mathrm{M} \) \( 1.400 \mathrm{M} \) \( 0.1000 \mathrm{M} \)
Expert Answer
The Concentration of NaOH (M1) = 0.1000 M From the analysis of graph the volume of NaOH at