Home /
Expert Answers /
Chemistry /
based-on-the-titration-curve-shown-below-determine-the-value-of-ka-titration-of-weak-acid-14-13-pa186
(Solved): Based on the titration curve shown below, determine the value of Ka Titration of Weak Acid 14- 13- ...
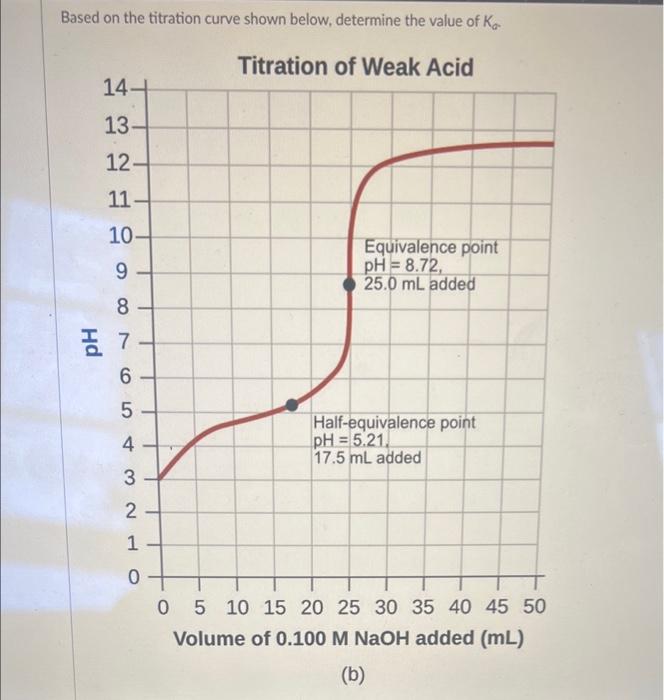

Based on the titration curve shown below, determine the value of Ka Titration of Weak Acid 14- 13- 12- 11- 10- 987 7 6 5. 432L 1 0 Equivalence point pH = 8.72, 25.0 mL added Half-equivalence point pH = 5.21. 17.5 mL added 0 5 10 15 20 25 30 35 40 45 50 Volume of 0.100 M NaOH added (mL) (b)
6.2 x 10-6 1.9 × 10-? 5.21 5.5 x 10-3
Expert Answer
Since , according to Henderson - Hasselbaclh equation : pH = pKa + log[salt]/[acid] at half equivalenc