Home /
Expert Answers /
Chemistry /
balance-the-reaction-au2s3-h2-au-h2s-what-is-the-coefficient-in-front-of-the-hydrogen-gas-pa528
(Solved): Balance the reaction: Au2S3 + H2 Au + H2S What is the coefficient in front of the hydrogen gas? ...


Balance the reaction: Au2S3 + H2 ? Au + H2S What is the coefficient in front of the hydrogen gas? A) 1 B) 2 C) 3 D) 4
Balance the reaction: CaCl2 + Na3PO4 ? Ca3(PO4)2 + NaCl What is the coefficient in front of the sodium phosphate? A) 1 B) 2 C)3 D) 6
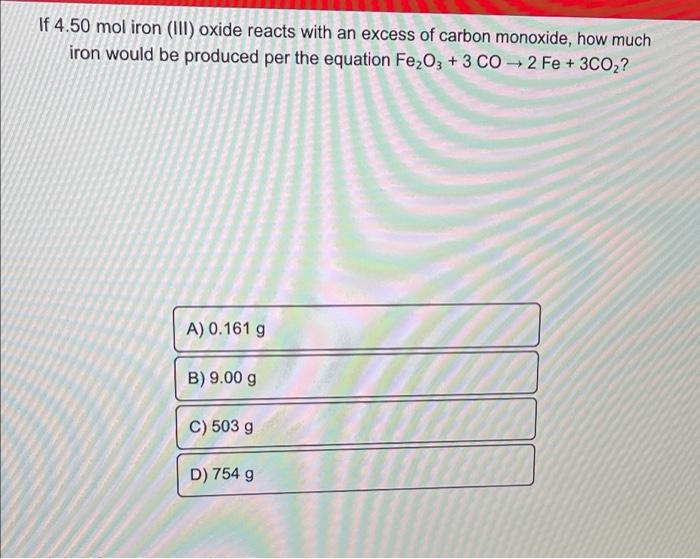
If 4.50 mol iron (III) oxide reacts with an excess of carbon monoxide, how much iron would be produced per the equation Fe2O3 + 3 CO2 Fe + 3CO2? A) 0.161 g B) 9.00 g C) 503 g W D) 754 g
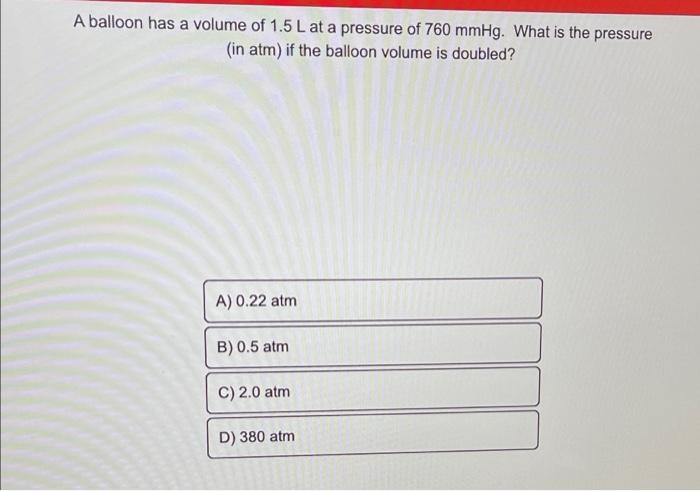
A balloon has a volume of 1.5 L at a pressure of 760 mmHg. What is the pressure (in atm) if the balloon volume is doubled? A) 0.22 atm B) 0.5 atm C) 2.0 atm D) 380 atm